Oxygen isotope biogeochemistry of pore water sulfate in the deep ...
Oxygen isotope biogeochemistry of pore water sulfate in the deep ...
Oxygen isotope biogeochemistry of pore water sulfate in the deep ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
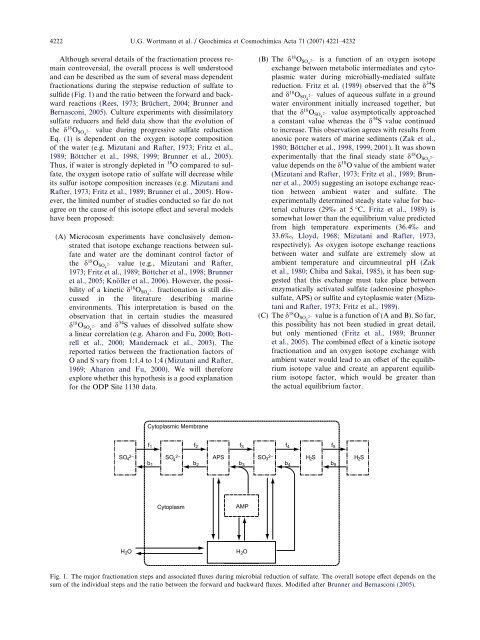
4222 U.G. Wortmann et al. / Geochimica et Cosmochimica Acta 71 (2007) 4221–4232Although several details <strong>of</strong> <strong>the</strong> fractionation process rema<strong>in</strong>controversial, <strong>the</strong> overall process is well understoodand can be described as <strong>the</strong> sum <strong>of</strong> several mass dependentfractionations dur<strong>in</strong>g <strong>the</strong> stepwise reduction <strong>of</strong> <strong>sulfate</strong> tosulfide (Fig. 1) and <strong>the</strong> ratio between <strong>the</strong> forward and backwardreactions (Rees, 1973; Brüchert, 2004; Brunner andBernasconi, 2005). Culture experiments with dissimilatory<strong>sulfate</strong> reducers and field data show that <strong>the</strong> evolution <strong>of</strong><strong>the</strong> d 18 O SO4 2 value dur<strong>in</strong>g progressive <strong>sulfate</strong> reductionEq. (1) is dependent on <strong>the</strong> oxygen <strong>isotope</strong> composition<strong>of</strong> <strong>the</strong> <strong>water</strong> (e.g. Mizutani and Rafter, 1973; Fritz et al.,1989; Böttcher et al., 1998, 1999; Brunner et al., 2005).Thus, if <strong>water</strong> is strongly depleted <strong>in</strong> 18 O compared to <strong>sulfate</strong>,<strong>the</strong> oxygen <strong>isotope</strong> ratio <strong>of</strong> <strong>sulfate</strong> will decrease whileits sulfur <strong>isotope</strong> composition <strong>in</strong>creases (e.g. Mizutani andRafter, 1973; Fritz et al., 1989; Brunner et al., 2005). However,<strong>the</strong> limited number <strong>of</strong> studies conducted so far do notagree on <strong>the</strong> cause <strong>of</strong> this <strong>isotope</strong> effect and several modelshave been proposed:(A) Microcosm experiments have conclusively demonstratedthat <strong>isotope</strong> exchange reactions between <strong>sulfate</strong>and <strong>water</strong> are <strong>the</strong> dom<strong>in</strong>ant control factor <strong>of</strong><strong>the</strong> d 18 O SO4 2 value (e.g., Mizutani and Rafter,1973; Fritz et al., 1989; Böttcher et al., 1998; Brunneret al., 2005; Knöller et al., 2006). However, <strong>the</strong> possibility<strong>of</strong> a k<strong>in</strong>etic d 18 O SO4 2 fractionation is still discussed<strong>in</strong> <strong>the</strong> literature describ<strong>in</strong>g mar<strong>in</strong>eenvironments. This <strong>in</strong>terpretation is based on <strong>the</strong>observation that <strong>in</strong> certa<strong>in</strong> studies <strong>the</strong> measuredd 18 O SO4 2 and d 34 S values <strong>of</strong> dissolved <strong>sulfate</strong> showa l<strong>in</strong>ear correlation (e.g. Aharon and Fu, 2000; Bottrellet al., 2000; Mandernack et al., 2003). Thereported ratios between <strong>the</strong> fractionation factors <strong>of</strong>O and S vary from 1:1.4 to 1:4 (Mizutani and Rafter,1969; Aharon and Fu, 2000). We will <strong>the</strong>reforeexplore whe<strong>the</strong>r this hypo<strong>the</strong>sis is a good explanationfor <strong>the</strong> ODP Site 1130 data.(B) The d 18 O SO4 2 is a function <strong>of</strong> an oxygen <strong>isotope</strong>exchange between metabolic <strong>in</strong>termediates and cytoplasmic<strong>water</strong> dur<strong>in</strong>g microbially-mediated <strong>sulfate</strong>reduction. Fritz et al. (1989) observed that <strong>the</strong> d 34 Sand d 18 O SO4 2 values <strong>of</strong> aqueous <strong>sulfate</strong> <strong>in</strong> a ground<strong>water</strong> environment <strong>in</strong>itially <strong>in</strong>creased toge<strong>the</strong>r, butthat <strong>the</strong> d 18 O SO4 2 value asymptotically approacheda constant value whereas <strong>the</strong> d 34 S value cont<strong>in</strong>uedto <strong>in</strong>crease. This observation agrees with results fromanoxic <strong>pore</strong> <strong>water</strong>s <strong>of</strong> mar<strong>in</strong>e sediments (Zak et al.,1980; Böttcher et al., 1998, 1999, 2001). It was shownexperimentally that <strong>the</strong> f<strong>in</strong>al steady state d 18 O SO4 2value depends on <strong>the</strong> d 18 O value <strong>of</strong> <strong>the</strong> ambient <strong>water</strong>(Mizutani and Rafter, 1973; Fritz et al., 1989; Brunneret al., 2005) suggest<strong>in</strong>g an <strong>isotope</strong> exchange reactionbetween ambient <strong>water</strong> and <strong>sulfate</strong>. Theexperimentally determ<strong>in</strong>ed steady state value for bacterialcultures (29‰ at 5 °C, Fritz et al., 1989) issomewhat lower than <strong>the</strong> equilibrium value predictedfrom high temperature experiments (36.4‰ and33.6‰, Lloyd, 1968; Mizutani and Rafter, 1973,respectively). As oxygen <strong>isotope</strong> exchange reactionsbetween <strong>water</strong> and <strong>sulfate</strong> are extremely slow atambient temperature and circumneutral pH (Zaket al., 1980; Chiba and Sakai, 1985), it has been suggestedthat this exchange must take place betweenenzymatically activated <strong>sulfate</strong> (adenos<strong>in</strong>e phospho<strong>sulfate</strong>,APS) or sulfite and cytoplasmic <strong>water</strong> (Mizutaniand Rafter, 1973; Fritz et al., 1989).(C) The d 18 O SO4 2 value is a function <strong>of</strong> (A and B). So far,this possibility has not been studied <strong>in</strong> great detail,but only mentioned (Fritz et al., 1989; Brunneret al., 2005). The comb<strong>in</strong>ed effect <strong>of</strong> a k<strong>in</strong>etic <strong>isotope</strong>fractionation and an oxygen <strong>isotope</strong> exchange withambient <strong>water</strong> would lead to an <strong>of</strong>fset <strong>of</strong> <strong>the</strong> equilibrium<strong>isotope</strong> value and create an apparent equilibrium<strong>isotope</strong> factor, which would be greater than<strong>the</strong> actual equilibrium factor.Cytoplasmic Membranef 1 f 2 f 3 f 4 f 5SO 2— 4 SO 2— 4 APSSO 2— 3 H 2 Sb 1 b 2 b 3 b 4 b 5H 2 SCytoplasmAMPH 2 OH 2 OFig. 1. The major fractionation steps and associated fluxes dur<strong>in</strong>g microbial reduction <strong>of</strong> <strong>sulfate</strong>. The overall <strong>isotope</strong> effect depends on <strong>the</strong>sum <strong>of</strong> <strong>the</strong> <strong>in</strong>dividual steps and <strong>the</strong> ratio between <strong>the</strong> forward and backward fluxes. Modified after Brunner and Bernasconi (2005).