Reaction Mechanisms of Charcoal and Coke in the ... - Pyro.co.za
Reaction Mechanisms of Charcoal and Coke in the ... - Pyro.co.za
Reaction Mechanisms of Charcoal and Coke in the ... - Pyro.co.za
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
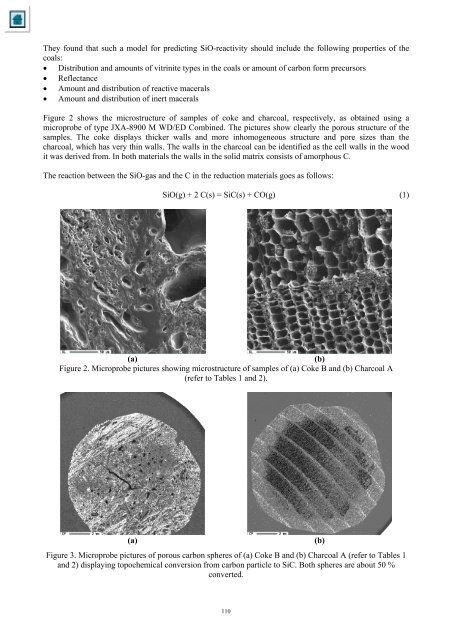
They found that such a model for predict<strong>in</strong>g SiO-reactivity should <strong>in</strong>clude <strong>the</strong> follow<strong>in</strong>g properties <strong>of</strong> <strong>the</strong><strong>co</strong>als:• Distribution <strong>and</strong> amounts <strong>of</strong> vitr<strong>in</strong>ite types <strong>in</strong> <strong>the</strong> <strong>co</strong>als or amount <strong>of</strong> carbon form precursors• Reflectance• Amount <strong>and</strong> distribution <strong>of</strong> reactive macerals• Amount <strong>and</strong> distribution <strong>of</strong> <strong>in</strong>ert maceralsFigure 2 shows <strong>the</strong> microstructure <strong>of</strong> samples <strong>of</strong> <strong>co</strong>ke <strong>and</strong> char<strong>co</strong>al, respectively, as obta<strong>in</strong>ed us<strong>in</strong>g amicroprobe <strong>of</strong> type JXA-8900 M WD/ED Comb<strong>in</strong>ed. The pictures show clearly <strong>the</strong> porous structure <strong>of</strong> <strong>the</strong>samples. The <strong>co</strong>ke displays thicker walls <strong>and</strong> more <strong>in</strong>homogeneous structure <strong>and</strong> pore sizes than <strong>the</strong>char<strong>co</strong>al, which has very th<strong>in</strong> walls. The walls <strong>in</strong> <strong>the</strong> char<strong>co</strong>al can be identified as <strong>the</strong> cell walls <strong>in</strong> <strong>the</strong> woodit was derived from. In both materials <strong>the</strong> walls <strong>in</strong> <strong>the</strong> solid matrix <strong>co</strong>nsists <strong>of</strong> amorphous C.The reaction between <strong>the</strong> SiO-gas <strong>and</strong> <strong>the</strong> C <strong>in</strong> <strong>the</strong> reduction materials goes as follows:SiO(g) + 2 C(s) = SiC(s) + CO(g) (1)(a)(b)Figure 2. Microprobe pictures show<strong>in</strong>g microstructure <strong>of</strong> samples <strong>of</strong> (a) <strong>Coke</strong> B <strong>and</strong> (b) <strong>Char<strong>co</strong>al</strong> A(refer to Tables 1 <strong>and</strong> 2).(a)Figure 3. Microprobe pictures <strong>of</strong> porous carbon spheres <strong>of</strong> (a) <strong>Coke</strong> B <strong>and</strong> (b) <strong>Char<strong>co</strong>al</strong> A (refer to Tables 1<strong>and</strong> 2) display<strong>in</strong>g topochemical <strong>co</strong>nversion from carbon particle to SiC. Both spheres are about 50 %<strong>co</strong>nverted.(b)