You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
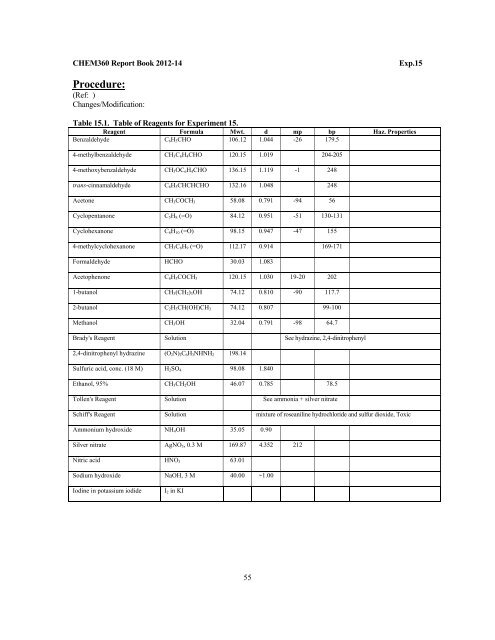
CHEM<strong>360</strong> Report Book 2012-14Exp.15Procedure:(Ref: )Changes/Modification:Table 15.1. Table of Reagents for Experiment 15.Reagent Formula Mwt. d mp bp Haz. PropertiesBenzaldehyde C 6 H 5 CHO 106.12 1.044 -26 179.54-methylbenzaldehyde CH 3 C 6 H 4 CHO 120.15 1.019 204-2054-methoxybenzaldehyde CH 3 OC 6 H 4 CHO 136.15 1.119 -1 248trans-cinnamaldehyde C 6 H 5 CHCHCHO 132.16 1.048 248Acetone CH 3 COCH 3 58.08 0.791 -94 56Cyclopentanone C 5 H 8 (=O) 84.12 0.951 -51 130-131Cyclohexanone C 6 H 10 (=O) 98.15 0.947 -47 1554-methylcyclohexanone CH 3 C 6 H 9 (=O) 112.17 0.914 169-171Formaldehyde HCHO 30.03 1.083Acetophenone C 6 H 5 COCH 3 120.15 1.030 19-20 2021-butanol CH 3 (CH 2 ) 3 OH 74.12 0.810 -90 117.72-butanol C 2 H 5 CH(OH)CH 3 74.12 0.807 99-100Methanol CH 3 OH 32.04 0.791 -98 64.7Brady's Reagent Solution See hydrazine, 2,4-dinitrophenyl2,4-dinitrophenyl hydrazine (O 2 N) 2 C 6 H 3 NHNH 2 198.14Sulfuric acid, conc. (18 M) H 2 SO 4 98.08 1.840Ethanol, 95% CH 3 CH 2 OH 46.07 0.785 78.5Tollen's Reagent Solution See ammonia + silver nitrateSchiff's Reagent Solution mixture of roseaniline hydrochloride and sulfur dioxide, ToxicAmmonium hydroxide NH 4 OH 35.05 0.90Silver nitrate AgNO 3 , 0.3 M 169.87 4.352 212Nitric acid HNO 3 63.01Sodium hydroxide NaOH, 3 M 40.00 ~1.00Iodine in potassium iodideI 2 in KI55