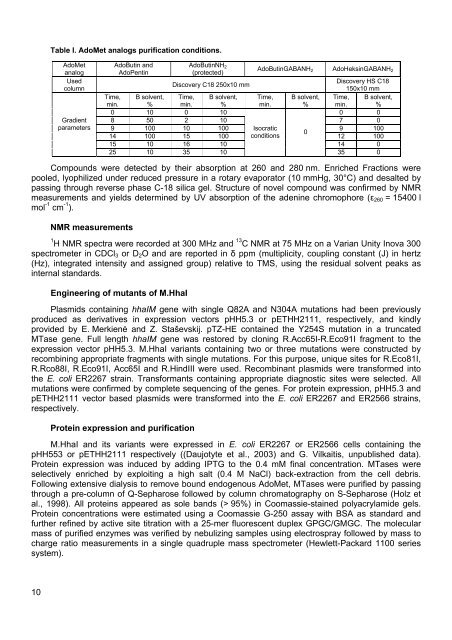
Table I. AdoMet analogs purification conditions.AdoMetanalogUsedcolumnGradientparametersAdoButin andAdoPentinAdoButinNH 2(protected)AdoButinGABANH 2 AdoHeksinGABANH 2Discovery C18 250x10 mmDiscovery HS C18150x10 mmTime,min.B solvent,%Time,min.B solvent,%Time,min.B solvent,%Time,min.B solvent,%0 10 0 10 0 08 50 2 10 7 09 100 10 100 Isocratic9 100014 100 15 100 conditions12 10015 10 16 10 14 025 10 35 1035 0Compounds were detected by their absorption at 260 and 280 nm. Enriched Fractions werepooled, lyophilized under reduced pressure in a rotary evaporator (10 mmHg, 30°C) and desalted bypassing through reverse phase C-18 silica gel. Structure of novel compound was confirmed by NMRmeasurements and yields determined by UV absorption of the adenine chromophore (ε 260 = 15400 lmol -1 cm -1 ).NMR measurements1H NMR spectra were recorded at 300 MHz and 13 C NMR at 75 MHz on a Varian Unity Inova 300spectrometer in CDCl 3 or D 2 O and are reported in δ ppm (multiplicity, coupling constant (J) in hertz(Hz), integrated intensity and assigned group) relative to TMS, using the residual solvent peaks asinternal standards.Engineering of mutants of M.HhaIPlasmids containing hhaIM gene with single Q82A and N304A mutations had been previouslyproduced as derivatives in expression vectors pHH5.3 or pETHH2111, respectively, and kindlyprovided by E. Merkienė and Z. Staševskij. pTZ-HE contained the Y254S mutation in a truncatedMTase gene. Full length hhaIM gene was restored by cloning R.Acc65I-R.Eco91I fragment to theexpression vector pHH5.3. M.HhaI variants containing two or three mutations were constructed byrecombining appropriate fragments with single mutations. For this purpose, unique sites for R.Eco81I,R.Rco88I, R.Eco91I, Acc65I and R.HindIII were used. Recombinant plasmids were transformed intothe E. coli ER2267 strain. Transformants containing appropriate diagnostic sites were selected. Allmutations were confirmed by complete sequencing of the genes. For protein expression, pHH5.3 andpETHH2111 vector based plasmids were transformed into the E. coli ER2267 and ER2566 strains,respectively.Protein expression and purificationM.HhaI and its variants were expressed in E. coli ER2267 or ER2566 cells containing thepHH553 or pETHH2111 respectively ((Daujotyte et al., 2003) and G. Vilkaitis, unpublished data).Protein expression was induced by adding IPTG to the 0.4 mM final concentration. MTases wereselectively enriched by exploiting a high salt (0.4 M NaCl) back-extraction from the cell debris.Following extensive dialysis to remove bound endogenous AdoMet, MTases were purified by passingthrough a pre-column of Q-Sepharose followed by column chromatography on S-Sepharose (Holz etal., 1998). All proteins appeared as sole bands (> 95%) in Coomassie-stained polyacrylamide gels.Protein concentrations were estimated using a Coomassie G-250 assay with BSA as standard andfurther refined by active site titration with a 25-mer fluorescent duplex GPGC/GMGC. The molecularmass of purified enzymes was verified by nebulizing samples using electrospray followed by mass tocharge ratio measurements in a single quadruple mass spectrometer (Hewlett-Packard 1100 seriessystem).10
DNA protection assay for alkyltransferase activityActivity of AdoMet analogs in the enzymatic reactions catalyzed by M.BcnIB or M.HhaI and itsvariants was tested on phage lambda DNA (215 M.HhaI recognition sites and 114 M.BcnIB recognitionsites). Analogous tests with M.Eco31I and M.BfiIC2 were performed on plasmid pUC19 DNA (1recognition site of M.Eco31I and 2 recognition sites of M.BfiC2).Twofold serial dilutions (15 µl) of DNA MTases starting with a stoichiometric ratio of DNA MTasesto recognition sites in buffer (M.HhaI/M.BcnIB buffer: 50 mM Tris-HCl, 10 mM NaCl, 0.5 mM EDTA, 2mM 2-mercaptoethanol, 0.2 mg/ml BSA, pH 7.4; M.Eco31I buffer: 20 mM MOPS, 20 mM Tris, 20 mMCAPS, 0.5 mM EDTA, 0.2 mg/ml BSA, pH 10.0; M.BfiIC2 buffer: 20 mM MOPS, 20 mM Tris, 20 mMCAPS, 0.5 mM EDTA, 0.2 mg/ml BSA, pH 9.0) containing phage lambda or plasmid pUC19 DNA andAdoMet analogs (total concentration of 300 µM) were incubated at 37°C (M.HhaI, M.BcnIB andM.Eco31I) or 30°C (M.BfiIC2) for 1 or 4 h. The reaction was stopped by heating at 80°C for 10 min. Incase of M.Eco31I and M.BfiIC2 pH of the samples was adjusted to 7.5 and 8.0 respectively by adding0.1 M HCl prior to restriction digestion. Afterwards a solution of restriction endonucleases (R.Hin6I toM.HhaI incubation, R.BcnI – M.BcnIB, R.Eco31I – M.Eco31I, R.BfiI – M.BfiC2, 2-10 u/1 µg DNA) andMgCl 2 (final concentration of 10 mM) was added to each sample and incubation was continued at37°C for 3 h. Reaction was stopped by adding proteinase K (0.2 mg/ml), SDS (0.1%) and heating at55°C for 30 min. Samples were supplemented with 1/6 volume of orange loading dye (0.2% orange G,0.05% xylene cyanol FF, 60% glycerol, 60 mM EDTA) and analyzed by agarose gel (1%)electrophoresis.Composition analysis of transalkylated DNAEnzymatic modifications with M.HhaI (Q82A or Q82A/N304A variants) and M.BcnIB (WT) wereperformed by incubation of an oligonucleotide duplex Uni#1.1:Uni#1.2 (10 µM) with AdoMet analogs(final concentration of 300 µM) and MTase (12.5 µM) in M.HhaI/M.BcnIB buffer (50 mM Tris-HCl, 10mM NaCl, 0.5 mM EDTA, 2 mM 2-mercaptoethanol, 0.2 mg/ml BSA, pH 7.4) at 37°C for 2-4 h.Samples were then incubated at 80°C for 10 min and treated with Proteinase K (0.2 mg/ml) and SDS(0.1%) at 55°C for 1 h. The modified oligodeoxynucleotides were desalted by gel filtration using G-25columns. Afterwards samples were supplemented with P1 buffer (10 mM Tris-HCl, 10 mM MgCl 2 , 1mM Zn(OAc) 2 , pH 7.5) containing Nuclease P1 (1.5 u) and calf intestine alkaline phosphatase (30 u)and incubated at 42°C for 4 h. Obtained hydrolysis solution was passed through a Microcon YM-3 spincolumn and subjected to LC/MS analysis.LC/MS analysisNucleosides or cofactor decomposition products were analyzed by reversed-phase HPLCcoupledelectrospray ionization mass spectrometry (Hewlett-Packard 1100 series system). Sampleswere loaded onto a column and eluted with linear gradient of two solvents A (20 mM HCOONH 4 , pH3.5) and B (80% methanol solution in water) at a flow of 0.3 ml/min (see table II). Separatedcompounds were detected by an in-line diode array UV absorbance detector at 210, 260 and 280 nm.Additionally, UV absorbance spectra were acquired (190-400 nm wavelength interval) at peak maximaand solvent contributions were removed by subtracting background spectra recorded before and afterthe peaks. For the mass spectrometric analysis of nucleosides post-column equal co-flow of 96%methanol, 4% formic acid and 1 mM sodium formate was used. For the MS detection of AdoMet, itsanalogs and decomposition products a post-column equal co-flow of 99% methanol, 1% CF 3 COOHwas used. Mass spectra were acquired in 50-800 m/z range in positive ion mode. Ionization capillaryvoltage was 5000 V, fragmentor voltage was in 100-120 V interval, drying gas temperature was 300-350°C and flow rate was 10-12 l/min.11