VILNIUS UNIVERSITY INSTITUTE OF BIOTECHNOLOGY ...
VILNIUS UNIVERSITY INSTITUTE OF BIOTECHNOLOGY ...
VILNIUS UNIVERSITY INSTITUTE OF BIOTECHNOLOGY ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
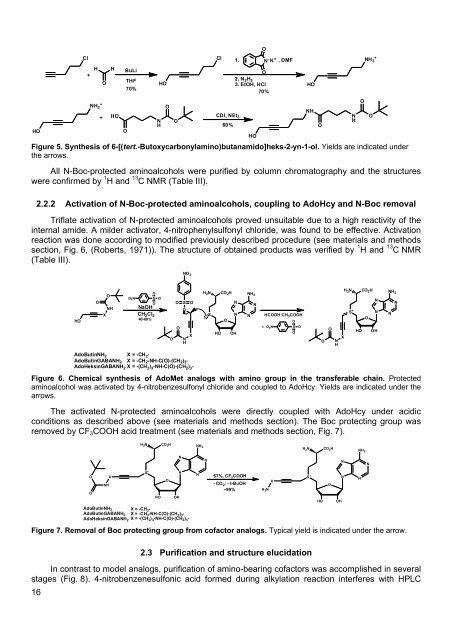
HOCl+HNH 3++OHHOBuLiTHFO70%NHHOOOCl1.CDI, NEt 360%ON - K + ,DMFO2. N 2 H 23. EtOH, HCl70%Figure 5. Synthesis of 6-[(tert.-Butoxycarbonylamino)butanamido]heks-2-yn-1-ol. Yields are indicated underthe arrows.All N-Boc-protected aminoalcohols were purified by column chromatography and the structureswere confirmed by 1 H and 13 C NMR (Table III).2.2.2 Activation of N-Boc-protected aminoalcohols, coupling to AdoHcy and N-Boc removalTriflate activation of N-protected aminoalcohols proved unsuitable due to a high reactivity of theinternal amide. A milder activator, 4-nitrophenylsulfonyl chloride, was found to be effective. Activationreaction was done according to modified previously described procedure (see materials and methodssection, Fig. 6, (Roberts, 1971)). The structure of obtained products was verified by 1 H and 13 C NMR(Table III).HOHONHONHONH 3+ONO 2HOOXONHO2NOSONaOHCH 2 Cl 240-60%ClOOOSONHOXH 2 NSCO 2 HNNOHO OHNH 2NN-HCOOH:CH 3 COOHOO 2 NS O -OOONHXH 2 NS +HOCO 2 HONNOHNH 2NNAdoButinNH 2 X=-CH 2 -AdoButinGABANH 2 X=-CH 2 -NH-C(O)-(CH 2 ) 3 -AdoHeksinGABANH 2 X=-(CH 2 ) 3 -NH-C(O)-(CH 2 ) 3 -Figure 6. Chemical synthesis of AdoMet analogs with amino group in the transferable chain. Protectedaminoalcohol was activated by 4-nitrobenzesulfonyl chloride and coupled to AdoHcy. Yields are indicated under thearrows.The activated N-protected aminoalcohols were directly coupled with AdoHcy under acidicconditions as described above (see materials and methods section). The Boc protecting group wasremoved by CF 3 COOH acid treatment (see materials and methods section, Fig. 7).H 2 NCO 2 HNH 2H 2 NCO 2 HNH 2NNNNS +NO XN 67%, CF 3 COOHONH-CO 2 ; - t-BuOHAdoHeksinGABANH 2 X=-(CH 2 ) 3 -NH-C(O)-(CH 2 ) 3 -~99%OHO OHAdoButinNH 2 X=-CH 2 -AdoButinGABANH 2 X=-CH 2 -NH-C(O)-(CH 2 ) 3 -Figure 7. Removal of Boc protecting group from cofactor analogs. Typical yield is indicated under the arrow.H 2 NXS +HOOOHNN162.3 Purification and structure elucidationIn contrast to model analogs, purification of amino-bearing cofactors was accomplished in severalstages (Fig. 8). 4-nitrobenzenesulfonic acid formed during alkylation reaction interferes with HPLC