VILNIUS UNIVERSITY INSTITUTE OF BIOTECHNOLOGY ...
VILNIUS UNIVERSITY INSTITUTE OF BIOTECHNOLOGY ...
VILNIUS UNIVERSITY INSTITUTE OF BIOTECHNOLOGY ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
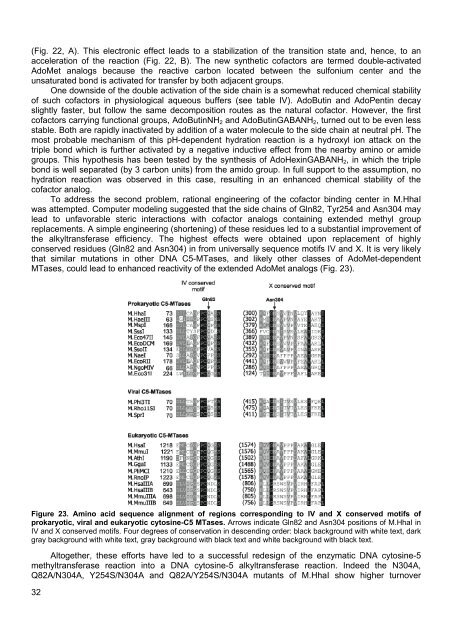
(Fig. 22, A). This electronic effect leads to a stabilization of the transition state and, hence, to anacceleration of the reaction (Fig. 22, B). The new synthetic cofactors are termed double-activatedAdoMet analogs because the reactive carbon located between the sulfonium center and theunsaturated bond is activated for transfer by both adjacent groups.One downside of the double activation of the side chain is a somewhat reduced chemical stabilityof such cofactors in physiological aqueous buffers (see table IV). AdoButin and AdoPentin decayslightly faster, but follow the same decomposition routes as the natural cofactor. However, the firstcofactors carrying functional groups, AdoButinNH 2 and AdoButinGABANH 2 , turned out to be even lessstable. Both are rapidly inactivated by addition of a water molecule to the side chain at neutral pH. Themost probable mechanism of this pH-dependent hydration reaction is a hydroxyl ion attack on thetriple bond which is further activated by a negative inductive effect from the nearby amino or amidegroups. This hypothesis has been tested by the synthesis of AdoHexinGABANH 2 , in which the triplebond is well separated (by 3 carbon units) from the amido group. In full support to the assumption, nohydration reaction was observed in this case, resulting in an enhanced chemical stability of thecofactor analog.To address the second problem, rational engineering of the cofactor binding center in M.HhaIwas attempted. Computer modeling suggested that the side chains of Gln82, Tyr254 and Asn304 maylead to unfavorable steric interactions with cofactor analogs containing extended methyl groupreplacements. A simple engineering (shortening) of these residues led to a substantial improvement ofthe alkyltransferase efficiency. The highest effects were obtained upon replacement of highlyconserved residues (Gln82 and Asn304) in from universally sequence motifs IV and X. It is very likelythat similar mutations in other DNA C5-MTases, and likely other classes of AdoMet-dependentMTases, could lead to enhanced reactivity of the extended AdoMet analogs (Fig. 23).Figure 23. Amino acid sequence alignment of regions corresponding to IV and X conserved motifs ofprokaryotic, viral and eukaryotic cytosine-C5 MTases. Arrows indicate Gln82 and Asn304 positions of M.HhaI inIV and X conserved motifs. Four degrees of conservation in descending order: black background with white text, darkgray background with white text, gray background with black text and white background with black text.Altogether, these efforts have led to a successful redesign of the enzymatic DNA cytosine-5methyltransferase reaction into a DNA cytosine-5 alkyltransferase reaction. Indeed the N304A,Q82A/N304A, Y254S/N304A and Q82A/Y254S/N304A mutants of M.HhaI show higher turnover32