Thermo Scientific Hypercarb Columns
Thermo Scientific Hypercarb Columns
Thermo Scientific Hypercarb Columns
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
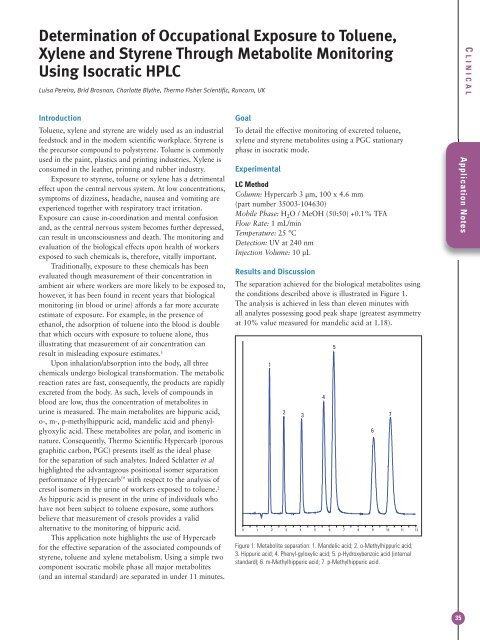
Determination of Occupational Exposure to Toluene,Xylene and Styrene Through Metabolite MonitoringUsing Isocratic HPLCLuisa Pereira, Brid Brosnan, Charlotte Blythe, <strong>Thermo</strong> Fisher <strong>Scientific</strong>, Runcorn, UKC LINICALIntroductionToluene, xylene and styrene are widely used as an industrialfeedstock and in the modern scientific workplace. Styrene isthe precursor compound to polystyrene. Toluene is commonlyused in the paint, plastics and printing industries. Xylene isconsumed in the leather, printing and rubber industry.Exposure to styrene, toluene or xylene has a detrimentaleffect upon the central nervous system. At low concentrations,symptoms of dizziness, headache, nausea and vomiting areexperienced together with respiratory tract irritation.Exposure can cause in-coordination and mental confusionand, as the central nervous system becomes further depressed,can result in unconsciousness and death. The monitoring andevaluation of the biological effects upon health of workersexposed to such chemicals is, therefore, vitally important.Traditionally, exposure to these chemicals has beenevaluated though measurement of their concentration inambient air where workers are more likely to be exposed to,however, it has been found in recent years that biologicalmonitoring (in blood or urine) affords a far more accurateestimate of exposure. For example, in the presence ofethanol, the adsorption of toluene into the blood is doublethat which occurs with exposure to toluene alone, thusillustrating that measurement of air concentration canresult in misleading exposure estimates. 1Upon inhalation/absorption into the body, all threechemicals undergo biological transformation. The metabolicreaction rates are fast, consequently, the products are rapidlyexcreted from the body. As such, levels of compounds inblood are low, thus the concentration of metabolites inurine is measured. The main metabolites are hippuric acid,o-, m-, p-methylhippuric acid, mandelic acid and phenylglyoxylicacid. These metabolites are polar, and isomeric innature. Consequently, <strong>Thermo</strong> <strong>Scientific</strong> <strong>Hypercarb</strong> (porousgraphitic carbon, PGC) presents itself as the ideal phasefor the separation of such analytes. Indeed Schlatter et alhighlighted the advantageous positional isomer separationperformance of <strong>Hypercarb</strong> with respect to the analysis ofcresol isomers in the urine of workers exposed to toluene. 2As hippuric acid is present in the urine of individuals whohave not been subject to toluene exposure, some authorsbelieve that measurement of cresols provides a validalternative to the monitoring of hippuric acid.This application note highlights the use of <strong>Hypercarb</strong>for the effective separation of the associated compounds ofstyrene, toluene and xylene metabolism. Using a simple twocomponent isocratic mobile phase all major metabolites(and an internal standard) are separated in under 11 minutes.GoalTo detail the effective monitoring of excreted toluene,xylene and styrene metabolites using a PGC stationaryphase in isocratic mode.ExperimentalLC MethodColumn: <strong>Hypercarb</strong> 3 µm, 100 x 4.6 mm(part number 35003-104630)Mobile Phase: H 2 O / MeOH (50:50) +0.1% TFAFlow Rate: 1 mL/minTemperature: 25 °CDetection: UV at 240 nmInjection Volume: 10 µLResults and DiscussionThe separation achieved for the biological metabolites usingthe conditions described above is illustrated in Figure 1.The analysis is achieved in less than eleven minutes withall analytes possessing good peak shape (greatest asymmetryat 10% value measured for mandelic acid at 1.18).Figure 1: Metabolite separation: 1. Mandelic acid; 2. o-Methylhippuric acid;3. Hippuric acid; 4. Phenyl-gyloxylic acid; 5. p-Hydroxybenzoic acid (internalstandard); 6. m-Methylhippuric acid; 7. p-Methylhippuric acid.Application Notes35