Thermo Scientific Hypercarb Columns
Thermo Scientific Hypercarb Columns
Thermo Scientific Hypercarb Columns
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
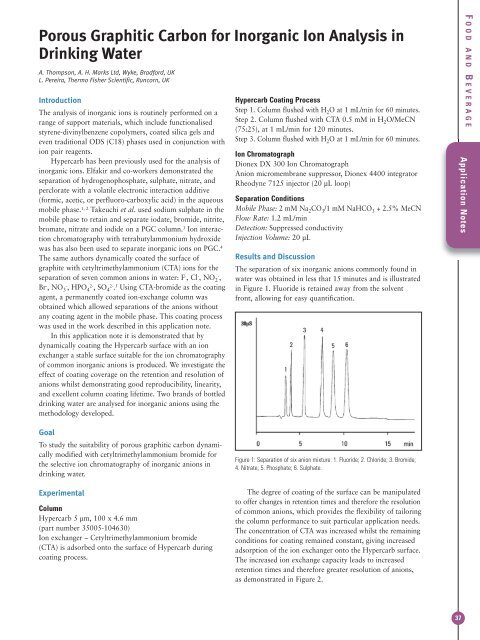
Porous Graphitic Carbon for Inorganic Ion Analysis inDrinking WaterA. Thompson, A. H. Marks Ltd, Wyke, Bradford, UKL. Pereira, <strong>Thermo</strong> Fisher <strong>Scientific</strong>, Runcorn, UKIntroductionThe analysis of inorganic ions is routinely performed on arange of support materials, which include functionalisedstyrene-divinylbenzene copolymers, coated silica gels andeven traditional ODS (C18) phases used in conjunction withion pair reagents.<strong>Hypercarb</strong> has been previously used for the analysis ofinorganic ions. Elfakir and co-workers demonstrated theseparation of hydrogenophosphate, sulphate, nitrate, andperclorate with a volatile electronic interaction additive(formic, acetic, or perfluoro-carboxylic acid) in the aqueousmobile phase. 1, 2 Takeuchi et al. used sodium sulphate in themobile phase to retain and separate iodate, bromide, nitrite,bromate, nitrate and iodide on a PGC column. 3 Ion interactionchromatography with tetrabutylammonium hydroxidewas has also been used to separate inorganic ions on PGC. 4The same authors dynamically coated the surface ofgraphite with cetyltrimethylammonium (CTA) ions for theseparation of seven common anions in water: F - , Cl - , NO 2-,Br - , NO 3-, HPO 42-, SO 42-. 5 Using CTA-bromide as the coatingagent, a permanently coated ion-exchange column wasobtained which allowed separations of the anions withoutany coating agent in the mobile phase. This coating processwas used in the work described in this application note.In this application note it is demonstrated that bydynamically coating the <strong>Hypercarb</strong> surface with an ionexchanger a stable surface suitable for the ion chromatographyof common inorganic anions is produced. We investigate theeffect of coating coverage on the retention and resolution ofanions whilst demonstrating good reproducibility, linearity,and excellent column coating lifetime. Two brands of bottleddrinking water are analysed for inorganic anions using themethodology developed.<strong>Hypercarb</strong> Coating ProcessStep 1. Column flushed with H 2 O at 1 mL/min for 60 minutes.Step 2. Column flushed with CTA 0.5 mM in H 2 O/MeCN(75:25), at 1 mL/min for 120 minutes.Step 3. Column flushed with H 2 O at 1 mL/min for 60 minutes.Ion ChromatographDionex DX 300 Ion ChromatographAnion micromembrane suppressor, Dionex 4400 integratorRheodyne 7125 injector (20 µL loop)Separation ConditionsMobile Phase: 2 mM Na 2 CO 3 /1 mM NaHCO 3 + 2.5% MeCNFlow Rate: 1.2 mL/minDetection: Suppressed conductivityInjection Volume: 20 µLResults and DiscussionThe separation of six inorganic anions commonly found inwater was obtained in less that 15 minutes and is illustratedin Figure 1. Fluoride is retained away from the solventfront, allowing for easy quantification.F OOD AND B EVERAGEApplication NotesGoalTo study the suitability of porous graphitic carbon dynamicallymodified with cetyltrimethylammonium bromide forthe selective ion chromatography of inorganic anions indrinking water.ExperimentalColumn<strong>Hypercarb</strong> 5 µm, 100 x 4.6 mm(part number 35005-104630)Ion exchanger – Cetyltrimethylammonium bromide(CTA) is adsorbed onto the surface of <strong>Hypercarb</strong> duringcoating process.Figure 1: Separation of six anion mixture: 1. Fluoride; 2. Chloride; 3. Bromide;4. Nitrate; 5. Phosphate; 6. Sulphate.The degree of coating of the surface can be manipulatedto offer changes in retention times and therefore the resolutionof common anions, which provides the flexibility of tailoringthe column performance to suit particular application needs.The concentration of CTA was increased whilst the remainingconditions for coating remained constant, giving increasedadsorption of the ion exchanger onto the <strong>Hypercarb</strong> surface.The increased ion exchange capacity leads to increasedretention times and therefore greater resolution of anions,as demonstrated in Figure 2.37