Introduction to Diffusion - Department of Materials Science and ...
Introduction to Diffusion - Department of Materials Science and ...
Introduction to Diffusion - Department of Materials Science and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
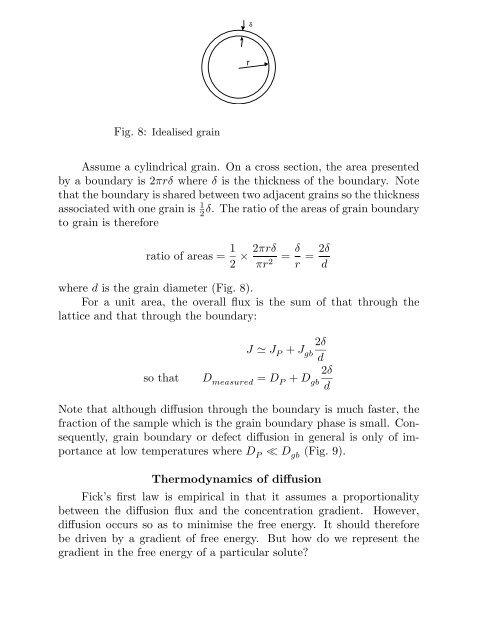
δrFig. 8: Idealised grainAssume a cylindrical grain. On a cross section, the area presentedby a boundary is 2πrδ where δ is the thickness <strong>of</strong> the boundary. Notethat the boundary is shared between two adjacent grains so the thicknessassociated with one grain is 1 2δ. The ratio <strong>of</strong> the areas <strong>of</strong> grain boundary<strong>to</strong> grain is thereforeratio <strong>of</strong> areas = 1 2 × 2πrδπr 2 = δ r = 2δdwhere d is the grain diameter (Fig. 8).For a unit area, the overall flux is the sum <strong>of</strong> that through thelattice <strong>and</strong> that through the boundary:so that2δJ ≃ J P + J gbd2δD measured = D P + D gbdNote that although diffusion through the boundary is much faster, thefraction <strong>of</strong> the sample which is the grain boundary phase is small. Consequently,grain boundary or defect diffusion in general is only <strong>of</strong> importanceat low temperatures where D P ≪ D gb (Fig. 9).Thermodynamics <strong>of</strong> diffusionFick’s first law is empirical in that it assumes a proportionalitybetween the diffusion flux <strong>and</strong> the concentration gradient. However,diffusion occurs so as <strong>to</strong> minimise the free energy. It should thereforebe driven by a gradient <strong>of</strong> free energy. But how do we represent thegradient in the free energy <strong>of</strong> a particular solute?