Introduction to Diffusion - Department of Materials Science and ...
Introduction to Diffusion - Department of Materials Science and ...
Introduction to Diffusion - Department of Materials Science and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
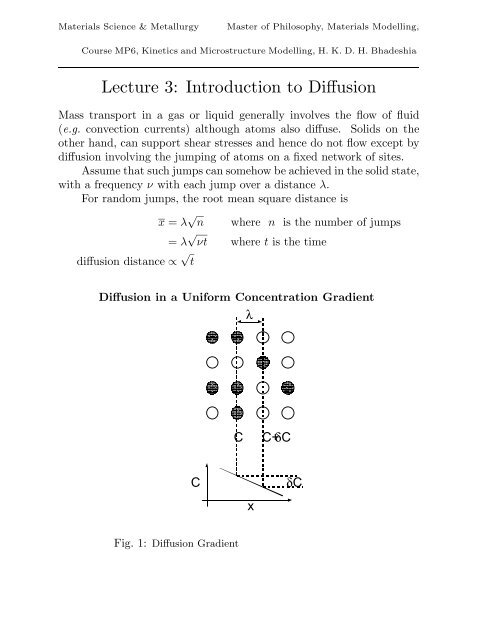
<strong>Materials</strong> <strong>Science</strong> & MetallurgyMaster <strong>of</strong> Philosophy, <strong>Materials</strong> Modelling,Course MP6, Kinetics <strong>and</strong> Microstructure Modelling, H. K. D. H. BhadeshiaLecture 3: <strong>Introduction</strong> <strong>to</strong> <strong>Diffusion</strong>Mass transport in a gas or liquid generally involves the flow <strong>of</strong> fluid(e.g. convection currents) although a<strong>to</strong>ms also diffuse. Solids on theother h<strong>and</strong>, can support shear stresses <strong>and</strong> hence do not flow except bydiffusion involving the jumping <strong>of</strong> a<strong>to</strong>ms on a fixed network <strong>of</strong> sites.Assume that such jumps can somehow be achieved in the solid state,with a frequency ν with each jump over a distance λ.For r<strong>and</strong>om jumps, the root mean square distance isx = λ √ ndiffusion distance ∝ √ twhere n is the number <strong>of</strong> jumps= λ √ νt where t is the time<strong>Diffusion</strong> in a Uniform Concentration GradientλCC+δCCxδCFig. 1: <strong>Diffusion</strong> Gradient
Concentration <strong>of</strong> solute, C, number m −3Each plane has Cλ a<strong>to</strong>ms m −2 (Fig. 1){ } ∂CδC = λ∂xA<strong>to</strong>mic flux, J, a<strong>to</strong>ms m −2 s −1J L→R = 1 6 νCλJ R→L = 1 ν(C + δC)λ6Therefore, the net flux along x is given byJ net = − 1 6 ν δC λ= − 1 { } ∂C6 ν λ2 ∂x{ } ∂C≡ −D∂xThis is Fick’s first law where the constant <strong>of</strong> proportionality is called thediffusion coefficient in m 2 s −1 . Fick’s first law applies <strong>to</strong> steady state fluxin a uniform concentration gradient. Thus, our equation for the me<strong>and</strong>iffusion distance can now be expressed in terms <strong>of</strong> the diffusivity asx = λ √ νt with D = 1 6 νλ2 giving x = √ 6Dt ≃ √ DtNon–Uniform Concentration GradientsSuppose that the concentration gradient is not uniform (Fig. 2).{ } ∂CFlux in = −D∂x1{ } ∂CFlux out = −D∂x2[{ } { ∂C ∂ 2 }]C= −D + δx∂x ∂x 21
unitareaδx1 2CxFig. 2: Non–uniform concentration gradientIn the time interval δt, the concentration changes δCδCδx = (Flux in – Flux out)δt∂C∂t = CD∂2 ∂x 2assuming that the diffusivity is independent <strong>of</strong> the concentration. Thisis Fick’s second law <strong>of</strong> diffusion.This is amenable <strong>to</strong> numerical solutions for the general case butthere are a couple <strong>of</strong> interesting analytical solutions for particular boundaryconditions. For a case where a fixed quantity <strong>of</strong> solute is plated on<strong>to</strong>a semi–infinite bar (Fig. 3),boundary conditions:C{x, t} =∫ ∞0C{x, t}dx = B<strong>and</strong> C{x, t = 0} = 0√ B { −x2expπDt 4DtNow imagine that we create the diffusion couple illustrated in Fig. 4,by stacking an infinite set <strong>of</strong> thin sources on the end <strong>of</strong> one <strong>of</strong> the bars.<strong>Diffusion</strong> can thus be treated by taking a whole set <strong>of</strong> the exponential}
Carea undereach curve isconstantx∞Fig. 3: Exponential solution. Note how the curvaturechanges with time.functions obtained above, each slightly displaced along the x axis, <strong>and</strong>summing (integrating) up their individual effects. The integral is in factthe error functionerf{x} = 2 √ π∫ xso the solution <strong>to</strong> the diffusion equation is0exp{−u 2 }duboundary conditions:C{x = 0, t} = C s<strong>and</strong> C{x, t = 0} = C 0{ } xC{x, t} = C s − (C s − C 0 )erf2 √ DtThis solution can be used in many circumstances where the surfaceconcentration is maintained constant, for example in the carburisationor decarburisation processes (the concentration pr<strong>of</strong>iles would be thesame as in Fig. 4, but with only one half <strong>of</strong> the couple illustrated). Thesolutions described here apply also <strong>to</strong> the diffusion <strong>of</strong> heat.Mechanism <strong>of</strong> <strong>Diffusion</strong>A<strong>to</strong>ms in the solid–state migrate by jumping in<strong>to</strong> vacancies (Fig. 5).The vacancies may be interstitial or in substitutional sites. There is,
C AC sC Bx∞∞Fig. 4: The error function solution. Notice that the“surface” concentration remains fixed.InterstitialSubstitutionalFig. 5: Mechanism <strong>of</strong> interstitial <strong>and</strong> substitutionaldiffusion.nevertheless, a barrier <strong>to</strong> the motion <strong>of</strong> the a<strong>to</strong>ms because the motionis associated with a transient dis<strong>to</strong>rtion <strong>of</strong> the lattice.Assuming that the a<strong>to</strong>m attempts jumps at a frequency ν 0 , thefrequency <strong>of</strong> successful jumps is given by}ν = ν 0 exp{− G∗kT{ }}S∗≡ ν 0 exp ×exp{− H∗k kT} {{ }independent <strong>of</strong> Twhere k <strong>and</strong> T are the Boltzmann constant <strong>and</strong> the absolute tempera-
ture respectively, <strong>and</strong> H ∗ <strong>and</strong> S ∗ the activation enthalpy <strong>and</strong> activationentropy respectively. SinceD ∝ ν we find D = D 0 exp{− H∗A plot <strong>of</strong> the logarithm <strong>of</strong> D versus 1/T should therefore give a straightline (Fig. 6), the slope <strong>of</strong> which is −H ∗ /k. Note that H ∗ is frequentlycalled the activation energy for diffusion <strong>and</strong> is <strong>of</strong>ten designated Q.kT}Fig. 6: Typical self–diffusion coefficients for puremetals <strong>and</strong> for carbon in ferritic iron. The uppermostdiffusivity for each metal is at its melting temperature.The activation enthalpy <strong>of</strong> diffusion can be separated in<strong>to</strong> two components,one the enthalpy <strong>of</strong> migration (due <strong>to</strong> dis<strong>to</strong>rtions) <strong>and</strong> the enthalpy<strong>of</strong> formation <strong>of</strong> a vacancy in an adjacent site. After all, for thea<strong>to</strong>m <strong>to</strong> jump it is necessary <strong>to</strong> have a vacant site; the equilibrium concentration<strong>of</strong> vacancies can be very small in solids. Since there are manymore interstitial vacancies, <strong>and</strong> since most interstitial sites are vacant,interstitial a<strong>to</strong>ms diffuse far more rapidly than substitutional solutes.Kirkendall Effect<strong>Diffusion</strong> is at first sight difficult <strong>to</strong> appreciate for the solid state. Anumber <strong>of</strong> mechanisms have been proposed his<strong>to</strong>rically. This includes avariety <strong>of</strong> ring mechanisms where a<strong>to</strong>ms simply swap positions, but controversyremained because the strain energies associated with such swapsmade the theories uncertain. One possibility is that diffusion occurs by
a<strong>to</strong>ms jumping in<strong>to</strong> vacancies. But the equilibrium concentration <strong>of</strong> vacanciesis typically 10 −6 , which is very small. The theory was thereforenot generally accepted until an elegant experiment by Smigelskas <strong>and</strong>Kirkendall (Fig. 7).Fig. 7: <strong>Diffusion</strong> couple with markersThe experiment applies <strong>to</strong> solids as well as cible liquids. Considera couple made from A <strong>and</strong> B. If the diffusion fluxes <strong>of</strong> the two elementsare different (|J A | > |J B |) then there will be a net flow <strong>of</strong> matter past theinert markers, causing the couple <strong>to</strong> shift bodily relative <strong>to</strong> the markers.This can only happen if diffusion is by a vacancy mechanism.An observer located at the markers will see not only a change inconcentration due <strong>to</strong> intrinsic diffusion, but also because <strong>of</strong> the Kirkendallflow <strong>of</strong> matter past the markers. The net effect is described bythe usual Fick’s laws, but with an interdiffusion coefficient D which isa weighted average <strong>of</strong> the two intrinsic diffusion coefficients:D = X B D A + X A D Bwhere X represents a mole fraction. It is the interdiffusion coefficientthat is measured in most experiments.Structure Sensitive <strong>Diffusion</strong>Crystals may contain nonequilibrium concentrations <strong>of</strong> defects suchas vacancies, dislocations <strong>and</strong> grain boundaries. These may provide easydiffusion paths through an otherwise perfect structure. Thus, the grainboundary diffusion coefficient D gb is expected <strong>to</strong> be much greater thanthe diffusion coefficient associated with the perfect structure, D P .
δrFig. 8: Idealised grainAssume a cylindrical grain. On a cross section, the area presentedby a boundary is 2πrδ where δ is the thickness <strong>of</strong> the boundary. Notethat the boundary is shared between two adjacent grains so the thicknessassociated with one grain is 1 2δ. The ratio <strong>of</strong> the areas <strong>of</strong> grain boundary<strong>to</strong> grain is thereforeratio <strong>of</strong> areas = 1 2 × 2πrδπr 2 = δ r = 2δdwhere d is the grain diameter (Fig. 8).For a unit area, the overall flux is the sum <strong>of</strong> that through thelattice <strong>and</strong> that through the boundary:so that2δJ ≃ J P + J gbd2δD measured = D P + D gbdNote that although diffusion through the boundary is much faster, thefraction <strong>of</strong> the sample which is the grain boundary phase is small. Consequently,grain boundary or defect diffusion in general is only <strong>of</strong> importanceat low temperatures where D P ≪ D gb (Fig. 9).Thermodynamics <strong>of</strong> diffusionFick’s first law is empirical in that it assumes a proportionalitybetween the diffusion flux <strong>and</strong> the concentration gradient. However,diffusion occurs so as <strong>to</strong> minimise the free energy. It should thereforebe driven by a gradient <strong>of</strong> free energy. But how do we represent thegradient in the free energy <strong>of</strong> a particular solute?
Dgbln{D}DPD measuredD gb2δd0.7T m1/TFig. 9: Structure sensitive diffusion. The dashed linewill in practice be curved.<strong>Diffusion</strong> in a Chemical Potential GradientFick’s laws are strictly empirical. <strong>Diffusion</strong> is driven by gradients<strong>of</strong> free energy rather than <strong>of</strong> chemical concentration:J A = −C A M A∂µ A∂xso thatD A = C A M A∂µ A∂C Awhere the proportionality constant M A is known as the mobility <strong>of</strong> A.In this equation, the diffusion coefficient is related <strong>to</strong> the mobility bycomparison with Fick’s first law. The chemical potential is here definedas the free energy per mole <strong>of</strong> A a<strong>to</strong>ms; it is necessary therefore<strong>to</strong> multiply by the concentration C A <strong>to</strong> obtain the actual free energygradient.The relationship is remarkable: if ∂µ A /∂C A > 0, then the diffusioncoefficient is positive <strong>and</strong> the chemical potential gradient is along thesame direction as the concentration gradient. However, if ∂µ A /∂C A < 0then the diffusion will occur against a concentration gradient!