Report - LifeSciences BC
Report - LifeSciences BC
Report - LifeSciences BC
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
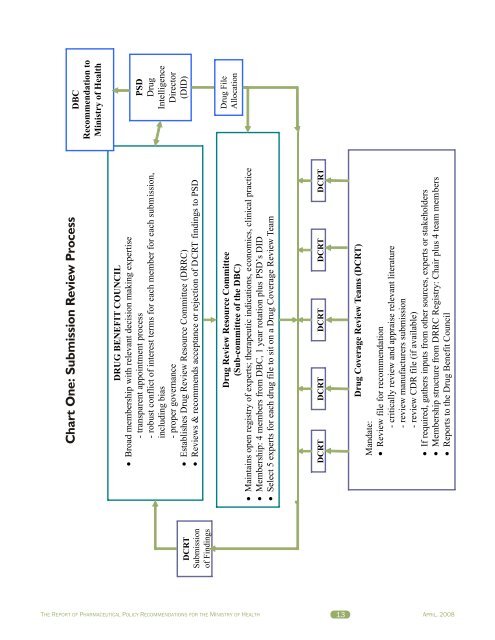
DCRTSubmissionof FindingsChart One: Submission Review ProcessDRUG BENEFIT COUNCILBroad membership with relevant decision making expertise- transparent appointment process- robust conflict of interest terms for each member for each submission,including bias- proper governanceEstablishes Drug Review Resource Committee (DRRC)Reviews & recommends acceptance or rejection of DCRT findings to PSDDrug Review Resource Committee(Sub-committee of the D<strong>BC</strong>)Maintains open registry of experts; therapeutic indications, economics, clinical practiceMembership: 4 members from D<strong>BC</strong>, 1 year rotation plus PSD’s DIDSelect 5 experts for each drug file to sit on a Drug Coverage Review TeamDCRT DCRT DCRT DCRT DCRTDrug Coverage Review Teams (DCRT)Mandate:Review file for recommendation- critically review and appraise relevant literature- review manufacturers submission- review CDR file (if available)If required, gathers inputs from other sources, experts or stakeholdersMembership structure from DRRC Registry: Chair plus 4 team members<strong>Report</strong>s to the Drug Benefit CouncilD<strong>BC</strong>Recommendation toMinistry of HealthPSDDrugIntelligenceDirector(DID)Drug FileAllocationTHE REPORT OF PHARMACEUTICAL POLICY RECOMMENDATIONS FOR THE MINISTRY OF HEALTH 13APRIL, 2008