Report - LifeSciences BC
Report - LifeSciences BC
Report - LifeSciences BC
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
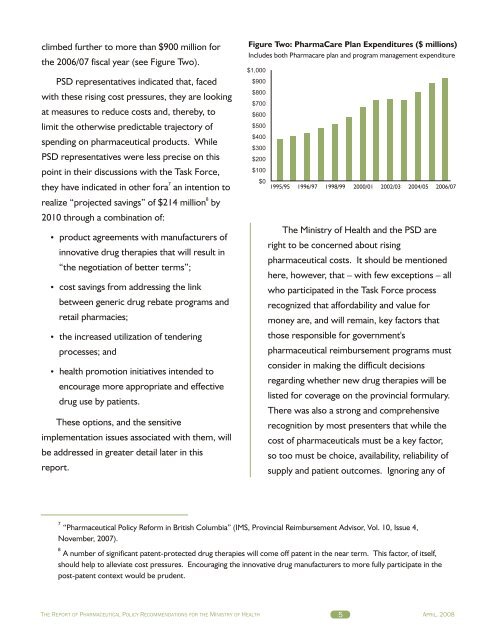
climbed further to more than $900 million forthe 2006/07 fiscal year (see Figure Two).PSD representatives indicated that, facedwith these rising cost pressures, they are lookingat measures to reduce costs and, thereby, tolimit the otherwise predictable trajectory ofspending on pharmaceutical products. WhilePSD representatives were less precise on thispoint in their discussions with the Task Force,7they have indicated in other fora an intention to8realize “projected savings” of $214 million by2010 through a combination of:product agreements with manufacturers ofinnovative drug therapies that will result in“the negotiation of better terms”;cost savings from addressing the linkbetween generic drug rebate programs andretail pharmacies;the increased utilization of tenderingprocesses; andhealth promotion initiatives intended toencourage more appropriate and effectivedrug use by patients.These options, and the sensitiveimplementation issues associated with them, willbe addressed in greater detail later in thisreport.Figure Two: PharmaCare Plan Expenditures ($ millions)Includes both Pharmacare plan and program management expenditure$1,000$900$800$700$600$500$400$300$200$100$01995/95 1996/97 1998/99 2000/01 2002/03 2004/05 2006/07The Ministry of Health and the PSD areright to be concerned about risingpharmaceutical costs. It should be mentionedhere, however, that – with few exceptions – allwho participated in the Task Force processrecognized that affordability and value formoney are, and will remain, key factors thatthose responsible for government'spharmaceutical reimbursement programs mustconsider in making the difficult decisionsregarding whether new drug therapies will belisted for coverage on the provincial formulary.There was also a strong and comprehensiverecognition by most presenters that while thecost of pharmaceuticals must be a key factor,so too must be choice, availability, reliability ofsupply and patient outcomes. Ignoring any of7“Pharmaceutical Policy Reform in British Columbia” (IMS, Provincial Reimbursement Advisor, Vol. 10, Issue 4,November, 2007).8A number of significant patent-protected drug therapies will come off patent in the near term. This factor, of itself,should help to alleviate cost pressures. Encouraging the innovative drug manufacturers to more fully participate in thepost-patent context would be prudent.THE REPORT OF PHARMACEUTICAL POLICY RECOMMENDATIONS FOR THE MINISTRY OF HEALTH 5APRIL, 2008