Monograph - Metrohm
Monograph - Metrohm
Monograph - Metrohm
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
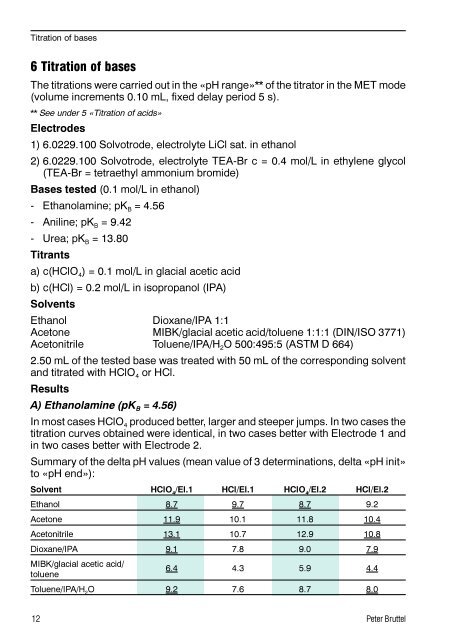
Titration of bases6 Titration of basesThe titrations were carried out in the «pH range»** of the titrator in the MET mode(volume increments 0.10 mL, fixed delay period 5 s).** See under 5 «Titration of acids»Electrodes1) 6.0229.100 Solvotrode, electrolyte LiCl sat. in ethanol2) 6.0229.100 Solvotrode, electrolyte TEA-Br c = 0.4 mol/L in ethylene glycol(TEA-Br = tetraethyl ammonium bromide)Bases tested (0.1 mol/L in ethanol)- Ethanolamine; pK B = 4.56- Aniline; pK B = 9.42- Urea; pK B = 13.80Titrantsa) c(HClO 4 ) = 0.1 mol/L in glacial acetic acidb) c(HCl) = 0.2 mol/L in isopropanol (IPA)SolventsEthanol Dioxane/IPA 1:1Acetone MIBK/glacial acetic acid/toluene 1:1:1 (DIN/ISO 3771)Acetonitrile Toluene/IPA/H 2 O 500:495:5 (ASTM D 664)2.50 mL of the tested base was treated with 50 mL of the corresponding solventand titrated with HClO 4 or HCl.ResultsA) Ethanolamine (pK B = 4.56)In most cases HClO 4 produced better, larger and steeper jumps. In two cases thetitration curves obtained were identical, in two cases better with Electrode 1 andin two cases better with Electrode 2.Summary of the delta pH values (mean value of 3 determinations, delta «pH init»to «pH end»):Solvent HClO 4/El.1 HCl/El.1 HClO 4/El.2 HCl/El.2Ethanol 8.7 9.7 8.7 9.2Acetone 11.9 10.1 11.8 10.4Acetonitrile 13.1 10.7 12.9 10.8Dioxane/IPA 9.1 7.8 9.0 7.9MIBK/glacial acetic acid/toluene6.4 4.3 5.9 4.4Toluene/IPA/H 2O 9.2 7.6 8.7 8.012 Peter Bruttel