Monograph - Metrohm
Monograph - Metrohm
Monograph - Metrohm
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
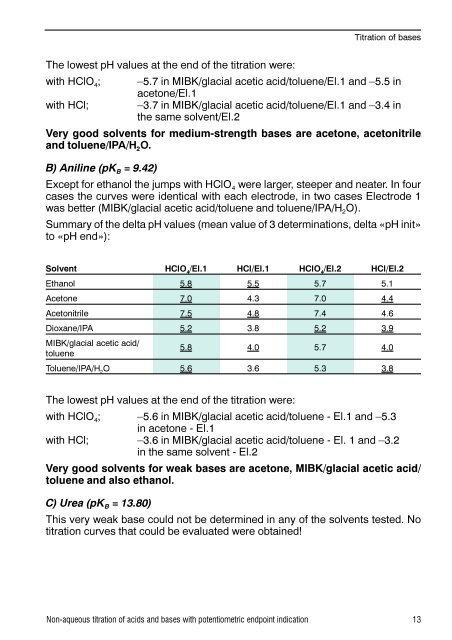
Titration of basesThe lowest pH values at the end of the titration were:with HClO 4 ; –5.7 in MIBK/glacial acetic acid/toluene/El.1 and –5.5 inacetone/El.1with HCl; –3.7 in MIBK/glacial acetic acid/toluene/El.1 and –3.4 inthe same solvent/El.2Very good solvents for medium-strength bases are acetone, acetonitrileand toluene/IPA/H 2 O.B) Aniline (pK B = 9.42)Except for ethanol the jumps with HClO 4 were larger, steeper and neater. In fourcases the curves were identical with each electrode, in two cases Electrode 1was better (MIBK/glacial acetic acid/toluene and toluene/IPA/H 2 O).Summary of the delta pH values (mean value of 3 determinations, delta «pH init»to «pH end»):Solvent HClO 4/El.1 HCl/El.1 HClO 4/El.2 HCl/El.2Ethanol 5.8 5.5 5.7 5.1Acetone 7.0 4.3 7.0 4.4Acetonitrile 7.5 4.8 7.4 4.6Dioxane/IPA 5.2 3.8 5.2 3.9MIBK/glacial acetic acid/toluene5.8 4.0 5.7 4.0Toluene/IPA/H 2O 5.6 3.6 5.3 3.8The lowest pH values at the end of the titration were:with HClO 4 ; –5.6 in MIBK/glacial acetic acid/toluene - El.1 and –5.3in acetone - El.1with HCl; –3.6 in MIBK/glacial acetic acid/toluene - El. 1 and –3.2in the same solvent - El.2Very good solvents for weak bases are acetone, MIBK/glacial acetic acid/toluene and also ethanol.C) Urea (pK B = 13.80)This very weak base could not be determined in any of the solvents tested. Notitration curves that could be evaluated were obtained!Non-aqueous titration of acids and bases with potentiometric endpoint indication 13