degree of unsaturation.†The hydrogen deficiency index is
degree of unsaturation.†The hydrogen deficiency index is
degree of unsaturation.†The hydrogen deficiency index is
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
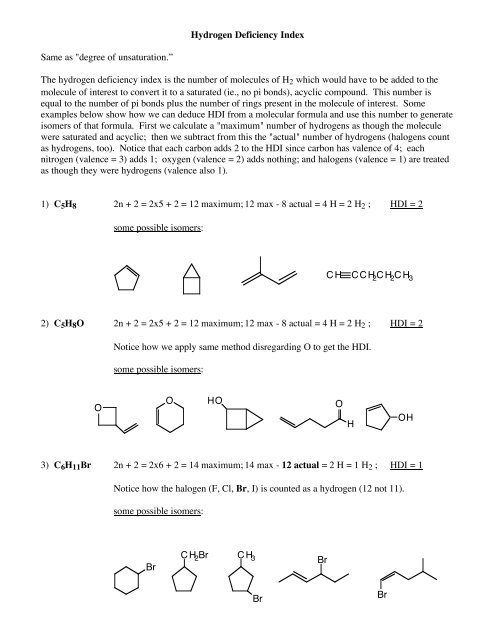
Hydrogen Deficiency IndexSame as "<strong>degree</strong> <strong>of</strong> <strong>unsaturation</strong>.”<strong>The</strong> <strong>hydrogen</strong> <strong>deficiency</strong> <strong>index</strong> <strong>is</strong> the number <strong>of</strong> molecules <strong>of</strong> H 2 which would have to be added to themolecule <strong>of</strong> interest to convert it to a saturated (ie., no pi bonds), acyclic compound. Th<strong>is</strong> number <strong>is</strong>equal to the number <strong>of</strong> pi bonds plus the number <strong>of</strong> rings present in the molecule <strong>of</strong> interest. Someexamples below show how we can deduce HDI from a molecular formula and use th<strong>is</strong> number to generate<strong>is</strong>omers <strong>of</strong> that formula. First we calculate a "maximum" number <strong>of</strong> <strong>hydrogen</strong>s as though the moleculewere saturated and acyclic; then we subtract from th<strong>is</strong> the "actual" number <strong>of</strong> <strong>hydrogen</strong>s (halogens countas <strong>hydrogen</strong>s, too). Notice that each carbon adds 2 to the HDI since carbon has valence <strong>of</strong> 4; eachnitrogen (valence = 3) adds 1; oxygen (valence = 2) adds nothing; and halogens (valence = 1) are treatedas though they were <strong>hydrogen</strong>s (valence also 1).1) C 5 H 8 2n + 2 = 2x5 + 2 = 12 maximum; 12 max - 8 actual = 4 H = 2 H 2 ; HDI = 2some possible <strong>is</strong>omers:C H C C H 2 C H 2 C H 32) C 5 H 8 O 2n + 2 = 2x5 + 2 = 12 maximum; 12 max - 8 actual = 4 H = 2 H 2 ; HDI = 2Notice how we apply same method d<strong>is</strong>regarding O to get the HDI.some possible <strong>is</strong>omers:OOHOOHOH3) C 6 H 11 Br 2n + 2 = 2x6 + 2 = 14 maximum; 14 max - 12 actual = 2 H = 1 H 2 ; HDI = 1Notice how the halogen (F, Cl, Br, I) <strong>is</strong> counted as a <strong>hydrogen</strong> (12 not 11).some possible <strong>is</strong>omers:BrC H 2 BrBrC H 3BrBr
4) C 4 H 9 N 2n + 2 + 1 = 2x4 + 2 + 1 = 11 maximum; 11 max - 9 actual = 2 H = 1 H 2 ; HDI = 1For each N present the maximum # <strong>of</strong> <strong>hydrogen</strong>s <strong>is</strong> increased by 1.some possible <strong>is</strong>omers:NHNH 2C H 2C HNHCH 3C HC HC H 3 2NCH 2 C H 35) C 6 H 11 ClN 2 O 2 <strong>The</strong>se rules hold simultaneously, <strong>of</strong> course:2n + 2 + 2 = 2x6 + 2 + 2 = 16 maximum; 16 max - 12 actual = 4 H = 2 H 2 ; HDI = 2some possible <strong>is</strong>omers:Cl OOOH 2 NNH 2H 2 NONH 2ClCl OClClOCO -NH 2ONHOHNNH+ 32In polycyclic compounds the number <strong>of</strong> rings <strong>is</strong> considered to be the minimum number <strong>of</strong> sigma bondsone must break to arrive at an acyclic structure; below are shown some examples where the dotted line <strong>is</strong>an imaginary bond cleavage:same as:same as:decalin;2 rings (bicyclic)HDI = 2; C 10 H 18norbornane;bicyclicHDI = 2; C 7 H 12norbornene;bicyclicHDI = 3; C 7 H 10adamantane;tricyclicHDI = 3; C 10 H 16