pH 7110 - Fagerberg
pH 7110 - Fagerberg
pH 7110 - Fagerberg
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
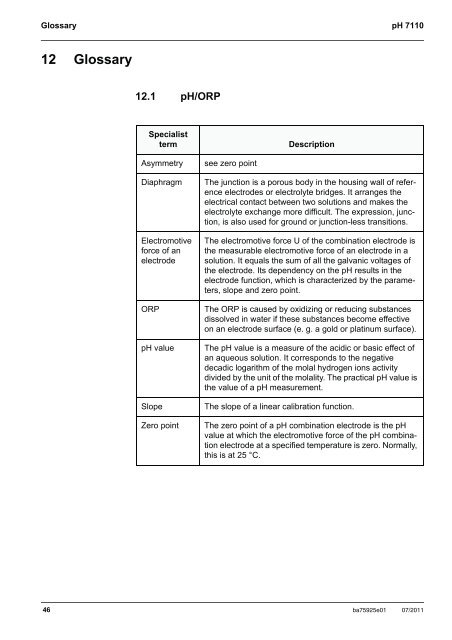
Glossary <strong>pH</strong> <strong>7110</strong>12 Glossary12.1 <strong>pH</strong>/ORPSpecialisttermDescriptionAsymmetryDiaphragmElectromotiveforce of anelectrodeORP<strong>pH</strong> valueSlopeZero pointsee zero pointThe junction is a porous body in the housing wall of referenceelectrodes or electrolyte bridges. It arranges theelectrical contact between two solutions and makes theelectrolyte exchange more difficult. The expression, junction,is also used for ground or junction-less transitions.The electromotive force U of the combination electrode isthe measurable electromotive force of an electrode in asolution. It equals the sum of all the galvanic voltages ofthe electrode. Its dependency on the <strong>pH</strong> results in theelectrode function, which is characterized by the parameters,slope and zero point.The ORP is caused by oxidizing or reducing substancesdissolved in water if these substances become effectiveon an electrode surface (e. g. a gold or platinum surface).The <strong>pH</strong> value is a measure of the acidic or basic effect ofan aqueous solution. It corresponds to the negativedecadic logarithm of the molal hydrogen ions activitydivided by the unit of the molality. The practical <strong>pH</strong> value isthe value of a <strong>pH</strong> measurement.The slope of a linear calibration function.The zero point of a <strong>pH</strong> combination electrode is the <strong>pH</strong>value at which the electromotive force of the <strong>pH</strong> combinationelectrode at a specified temperature is zero. Normally,this is at 25 °C.46 ba75925e01 07/2011