PDE4inibitors Cochrane 2011.pdf
PDE4inibitors Cochrane 2011.pdf
PDE4inibitors Cochrane 2011.pdf
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
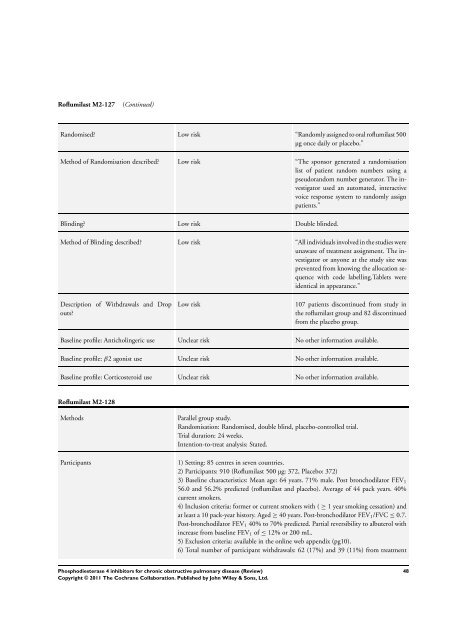
Roflumilast M2-127(Continued)Randomised? Low risk “Randomly assigned to oral roflumilast 500µg once daily or placebo.”Method of Randomisation described? Low risk “The sponsor generated a randomisationlist of patient random numbers using apseudorandom number generator. The investigatorused an automated, interactivevoice response system to randomly assignpatients.”Blinding? Low risk Double blinded.Method of Blinding described? Low risk “All individuals involved in the studies wereunaware of treatment assignment. The investigatoror anyone at the study site wasprevented from knowing the allocation sequencewith code labelling.Tablets wereidentical in appearance.”Description of Withdrawals and Dropouts?Low risk107 patients discontinued from study inthe roflumilast group and 82 discontinuedfrom the placebo group.Baseline profile: Anticholingeric use Unclear risk No other information available.Baseline profile: β2 agonist use Unclear risk No other information available.Baseline profile: Corticosteroid use Unclear risk No other information available.Roflumilast M2-128MethodsParticipantsParallel group study.Randomisation: Randomised, double blind, placebo-controlled trial.Trial duration: 24 weeks.Intention-to-treat analysis: Stated.1) Setting: 85 centres in seven countries.2) Participants: 910 (Roflumilast 500 µg: 372, Placebo: 372)3) Baseline characteristics: Mean age: 64 years. 71% male. Post bronchodilator FEV 156.0 and 56.2% predicted (roflumilast and placebo). Average of 44 pack years. 40%current smokers.4) Inclusion criteria: former or current smokers with ( ≥ 1 year smoking cessation) andat least a 10 pack-year history. Aged ≥ 40 years. Post-bronchodilator FEV 1 /FVC ≤ 0.7.Post-bronchodilator FEV 1 40% to 70% predicted. Partial reversibility to albuterol withincrease from baseline FEV 1 of ≤ 12% or 200 mL.5) Exclusion criteria: available in the online web appendix (pg10).6) Total number of participant withdrawals: 62 (17%) and 39 (11%) from treatmentPhosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease (Review)Copyright © 2011 The <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.48