pH = 11 - SKB
pH = 11 - SKB
pH = 11 - SKB
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
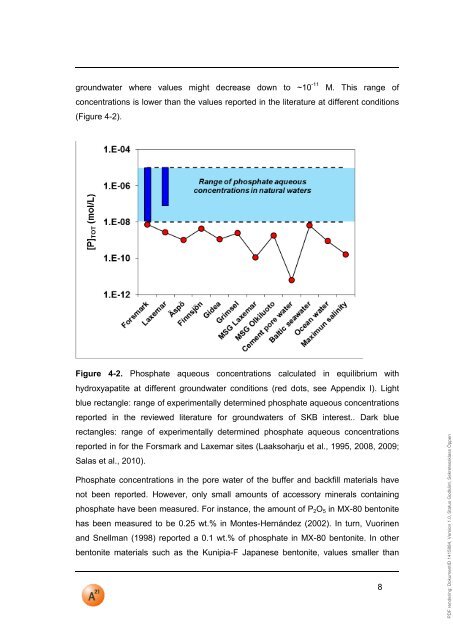
PDF rendering: DokumentID 1415884, Version 1.0, Status Godkänt, Sekretessklass Öppengroundwater where values might decrease down to ~10 -<strong>11</strong> M. This range ofconcentrations is lower than the values reported in the literature at different conditions(Figure 4-2).Figure 4-2. Phosphate aqueous concentrations calculated in equilibrium withhydroxyapatite at different groundwater conditions (red dots, see Appendix I). Lightblue rectangle: range of experimentally determined phosphate aqueous concentrationsreported in the reviewed literature for groundwaters of <strong>SKB</strong> interest.. Dark bluerectangles: range of experimentally determined phosphate aqueous concentrationsreported in for the Forsmark and Laxemar sites (Laaksoharju et al., 1995, 2008, 2009;Salas et al., 2010).Phosphate concentrations in the pore water of the buffer and backfill materials havenot been reported. However, only small amounts of accessory minerals containingphosphate have been measured. For instance, the amount of P 2 O 5 in MX-80 bentonitehas been measured to be 0.25 wt.% in Montes-Hernández (2002). In turn, Vuorinenand Snellman (1998) reported a 0.1 wt.% of phosphate in MX-80 bentonite. In otherbentonite materials such as the Kunipia-F Japanese bentonite, values smaller than8