pH = 11 - SKB
pH = 11 - SKB
pH = 11 - SKB
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
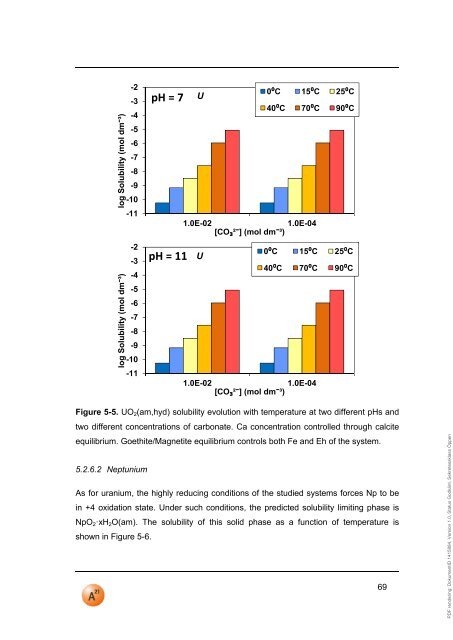
PDF rendering: DokumentID 1415884, Version 1.0, Status Godkänt, Sekretessklass Öppenlog Solubility (mol dm−³)log Solubility (mol dm−³)-2-3-4-5-6-7-8-9-10-<strong>11</strong>-2-3-4-5-6-7-8-9-10-<strong>11</strong><strong>pH</strong> = 7<strong>pH</strong> = <strong>11</strong>U0⁰C 15⁰C 25⁰C40⁰C 70⁰C 90⁰C1.0E-021.0E-04[CO₃²−] (mol dm−³)U0⁰C 15⁰C 25⁰C40⁰C 70⁰C 90⁰C1.0E-021.0E-04[CO₃²−] (mol dm−³)Figure 5-5. UO 2 (am,hyd) solubility evolution with temperature at two different <strong>pH</strong>s andtwo different concentrations of carbonate. Ca concentration controlled through calciteequilibrium. Goethite/Magnetite equilibrium controls both Fe and Eh of the system.5.2.6.2 NeptuniumAs for uranium, the highly reducing conditions of the studied systems forces Np to bein +4 oxidation state. Under such conditions, the predicted solubility limiting phase isNpO 2·xH 2 O(am). The solubility of this solid phase as a function of temperature isshown in Figure 5-6.69