pH = 11 - SKB
pH = 11 - SKB
pH = 11 - SKB
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
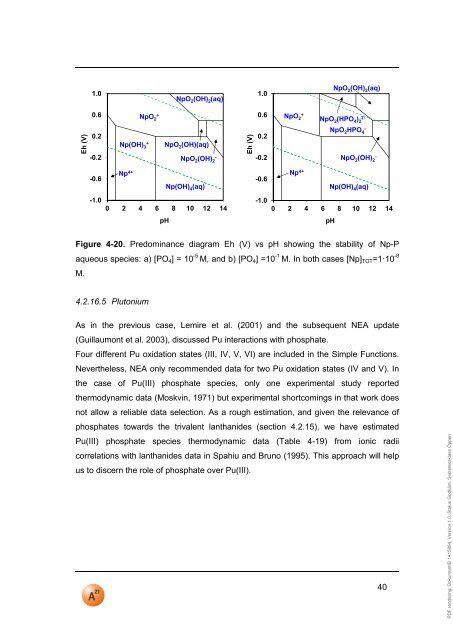
PDF rendering: DokumentID 1415884, Version 1.0, Status Godkänt, Sekretessklass Öppen1.0NpO 2 (OH) 2 (aq)1.0NpO 2 (OH) 2 (aq)0.6NpO 2+0.6NpO 2+NpO 2 (HPO 4 ) 22-Eh (V)0.2-0.2-0.6Np(OH) 3+Np 4+NpO 2 (OH)(aq)NpO 2 (OH)-2Np(OH) 4 (aq)Eh (V)0.2-0.2-0.6NpO 2 HPO- 4NpO 2 (OH)- 2Np 4+ Np(OH) 4 (aq)-1.00 2 4 6 8 10 12 14-1.00 2 4 6 8 10 12 14<strong>pH</strong><strong>pH</strong>Figure 4-20. Predominance diagram Eh (V) vs <strong>pH</strong> showing the stability of Np-Paqueous species: a) [PO 4 ] = 10 -5 M, and b) [PO 4 ] =10 -1 M. In both cases [Np] TOT =1·10 -8M.4.2.16.5 PlutoniumAs in the previous case, Lemire et al. (2001) and the subsequent NEA update(Guillaumont et al. 2003), discussed Pu interactions with phosphate.Four different Pu oxidation states (III, IV, V, VI) are included in the Simple Functions.Nevertheless, NEA only recommended data for two Pu oxidation states (IV and V). Inthe case of Pu(III) phosphate species, only one experimental study reportedthermodynamic data (Moskvin, 1971) but experimental shortcomings in that work doesnot allow a reliable data selection. As a rough estimation, and given the relevance ofphosphates towards the trivalent lanthanides (section 4.2.15), we have estimatedPu(III) phosphate species thermodynamic data (Table 4-19) from ionic radiicorrelations with lanthanides data in Spahiu and Bruno (1995). This approach will helpus to discern the role of phosphate over Pu(III).40