pH = 11 - SKB
pH = 11 - SKB
pH = 11 - SKB
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
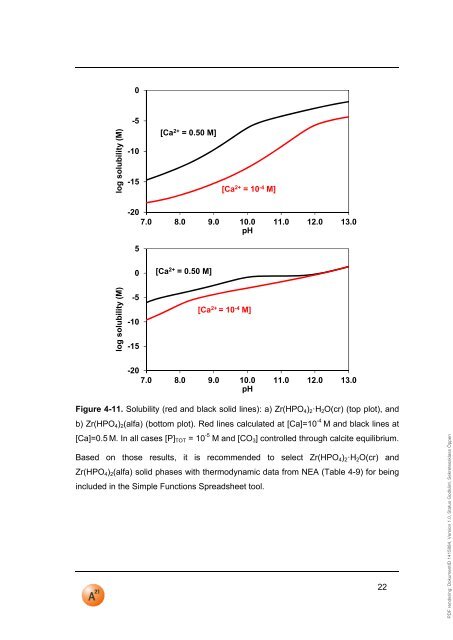
PDF rendering: DokumentID 1415884, Version 1.0, Status Godkänt, Sekretessklass Öppen0log solubility (M)-5-10-15[Ca 2+ = 0.50 M][Ca 2+ = 10 -4 M]-207.0 8.0 9.0 10.0 <strong>11</strong>.0 12.0 13.0<strong>pH</strong>50[Ca 2+ = 0.50 M]log solubility (M)-5-10-15[Ca 2+ = 10 -4 M]-207.0 8.0 9.0 10.0 <strong>11</strong>.0 12.0 13.0<strong>pH</strong>Figure 4-<strong>11</strong>. Solubility (red and black solid lines): a) Zr(HPO 4 ) 2·H 2 O(cr) (top plot), andb) Zr(HPO 4 ) 2 (alfa) (bottom plot). Red lines calculated at [Ca]=10 -4 M and black lines at[Ca]=0.5 M. In all cases [P] TOT = 10 -5 M and [CO 3 ] controlled through calcite equilibrium.Based on those results, it is recommended to select Zr(HPO 4 ) 2·H 2 O(cr) andZr(HPO 4 ) 2 (alfa) solid phases with thermodynamic data from NEA (Table 4-9) for beingincluded in the Simple Functions Spreadsheet tool.22