pH = 11 - SKB
pH = 11 - SKB
pH = 11 - SKB
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
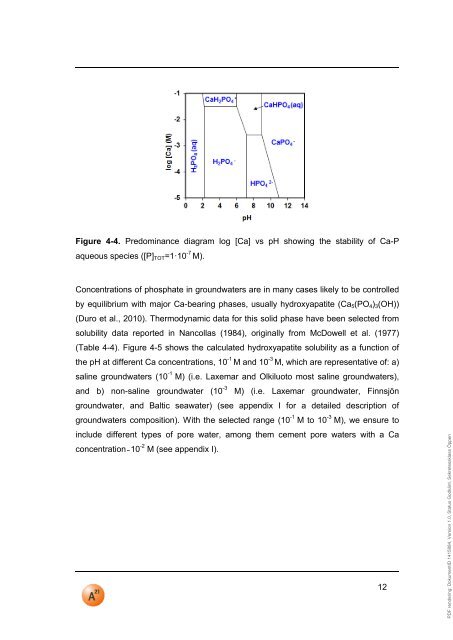
PDF rendering: DokumentID 1415884, Version 1.0, Status Godkänt, Sekretessklass ÖppenFigure 4-4. Predominance diagram log [Ca] vs <strong>pH</strong> showing the stability of Ca-Paqueous species ([P] TOT =1·10 -7 M).Concentrations of phosphate in groundwaters are in many cases likely to be controlledby equilibrium with major Ca-bearing phases, usually hydroxyapatite (Ca 5 (PO 4 ) 3 (OH))(Duro et al., 2010). Thermodynamic data for this solid phase have been selected fromsolubility data reported in Nancollas (1984), originally from McDowell et al. (1977)(Table 4-4). Figure 4-5 shows the calculated hydroxyapatite solubility as a function ofthe <strong>pH</strong> at different Ca concentrations, 10 -1 M and 10 -3 M, which are representative of: a)saline groundwaters (10 -1 M) (i.e. Laxemar and Olkiluoto most saline groundwaters),and b) non-saline groundwater (10 -3 M) (i.e. Laxemar groundwater, Finnsjöngroundwater, and Baltic seawater) (see appendix I for a detailed description ofgroundwaters composition). With the selected range (10 -1 M to 10 -3 M), we ensure toinclude different types of pore water, among them cement pore waters with a Caconcentration ̴ 10 -2 M (see appendix I).12