pH = 11 - SKB
pH = 11 - SKB
pH = 11 - SKB
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
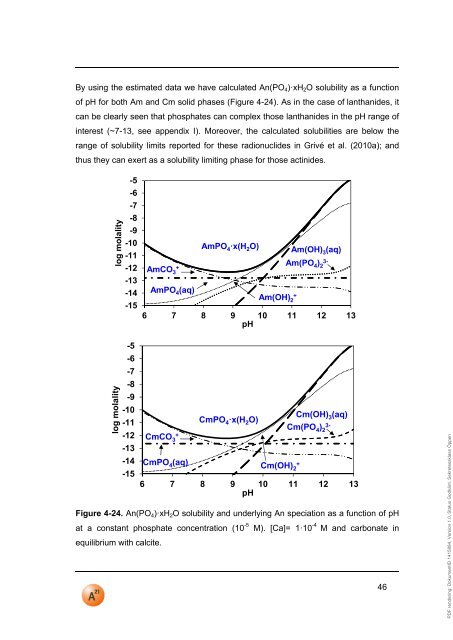
PDF rendering: DokumentID 1415884, Version 1.0, Status Godkänt, Sekretessklass ÖppenBy using the estimated data we have calculated An(PO 4 )·xH 2 O solubility as a functionof <strong>pH</strong> for both Am and Cm solid phases (Figure 4-24). As in the case of lanthanides, itcan be clearly seen that phosphates can complex those lanthanides in the <strong>pH</strong> range ofinterest (~7-13, see appendix I). Moreover, the calculated solubilities are below therange of solubility limits reported for these radionuclides in Grivé et al. (2010a); andthus they can exert as a solubility limiting phase for those actinides.log molalitylog molality-5-6-7-8-9-10-<strong>11</strong>-12-13-14-15-5-6-7-8-9-10-<strong>11</strong>-12-13-14-15AmPO 4·x(H 2 O) Am(OH) 3 (aq)AmCO+3Am(PO 4 )3-2AmPO 4 (aq)Am(OH)+26 7 8 9 10 <strong>11</strong> 12 13<strong>pH</strong>Cm(OH)CmPO 4·x(H 2 O)3 (aq)Cm(PO 4 )3-2CmCO+3CmPO 4 (aq)Cm(OH)+26 7 8 9 10 <strong>11</strong> 12 13<strong>pH</strong>Figure 4-24. An(PO 4 )·xH 2 O solubility and underlying An speciation as a function of <strong>pH</strong>at a constant phosphate concentration (10 -5 M). [Ca]= 1·10 -4 M and carbonate inequilibrium with calcite.46