Netherlands Journal
NJCC Volume 10, Oktober 2006
NJCC Volume 10, Oktober 2006
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
netherlands journal of critical care<br />
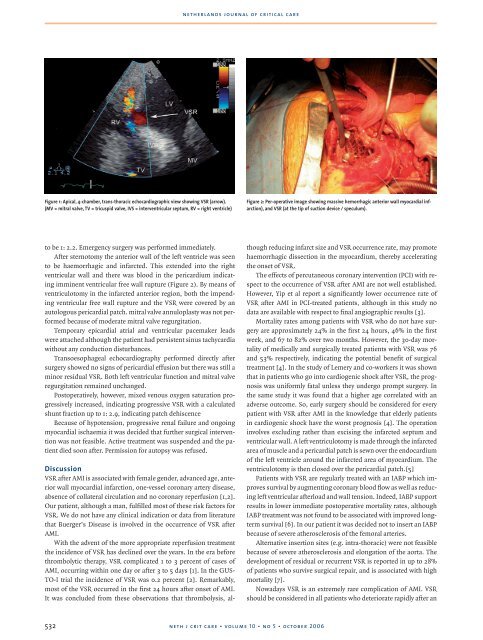
Figure 1: Apical, 4-chamber, trans-thoracic echocardiographic view showing VSR (arrow).<br />
(MV = mitral valve, TV = tricuspid valve, IVS = interventricular septum, RV = right ventricle)<br />
Figure 2: Per-operative image showing massive hemorrhagic anterior wall myocardial infarction),<br />
and VSR (at the tip of suction device / speculum).<br />
to be 1: 2.2. Emergency surgery was performed immediately.<br />
After sternotomy the anterior wall of the left ventricle was seen<br />
to be haemorrhagic and infarcted. This extended into the right<br />
ventricular wall and there was blood in the pericardium indicating<br />
imminent ventricular free wall rupture (Figure 2). By means of<br />
ventriculotomy in the infarcted anterior region, both the impending<br />
ventricular free wall rupture and the VSR were covered by an<br />
autologous pericardial patch. mitral valve annuloplasty was not performed<br />
because of moderate mitral valve regurgitation.<br />
Temporary epicardial atrial and ventricular pacemaker leads<br />
were attached although the patient had persistent sinus tachycardia<br />
without any conduction disturbances.<br />
Transoesophageal echocardiography performed directly after<br />
surgery showed no signs of pericardial effusion but there was still a<br />
minor residual VSR. Both left ventricular function and mitral valve<br />
regurgitation remained unchanged.<br />
Postoperatively, however, mixed venous oxygen saturation progressively<br />
increased, indicating progressive VSR with a calculated<br />
shunt fraction up to 1: 2.9, indicating patch dehiscence<br />
Because of hypotension, progressive renal failure and ongoing<br />
myocardial ischaemia it was decided that further surgical intervention<br />
was not feasible. Active treatment was suspended and the patient<br />
died soon after. Permission for autopsy was refused.<br />
Discussion<br />
VSR after AMI is associated with female gender, advanced age, anterior<br />
wall myocardial infarction, one-vessel coronary artery disease,<br />
absence of collateral circulation and no coronary reperfusion [1,2].<br />
Our patient, although a man, fulfilled most of these risk factors for<br />
VSR. We do not have any clinical indication or data from literature<br />
that Buerger’s Disease is involved in the occurrence of VSR after<br />
AMI.<br />
With the advent of the more appropriate reperfusion treatment<br />
the incidence of VSR has declined over the years. In the era before<br />
thrombolytic therapy, VSR complicated 1 to 3 percent of cases of<br />
AMI, occurring within one day or after 3 to 5 days [1]. In the GUS-<br />
TO-I trial the incidence of VSR was 0.2 percent [2]. Remarkably,<br />
most of the VSR occurred in the first 24 hours after onset of AMI.<br />
It was concluded from these observations that thrombolysis, although<br />
reducing infarct size and VSR occurrence rate, may promote<br />
haemorrhagic dissection in the myocardium, thereby accelerating<br />
the onset of VSR.<br />
The effects of percutaneous coronary intervention (PCI) with respect<br />
to the occurrence of VSR after AMI are not well established.<br />
However, Yip et al report a significantly lower occurrence rate of<br />
VSR after AMI in PCI-treated patients, although in this study no<br />
data are available with respect to final angiographic results [3].<br />
Mortality rates among patients with VSR who do not have surgery<br />
are approximately 24% in the first 24 hours, 46% in the first<br />
week, and 67 to 82% over two months. However, the 30-day mortality<br />
of medically and surgically treated patients with VSR was 76<br />
and 53% respectively, indicating the potential benefit of surgical<br />
treatment [4]. In the study of Lemery and co-workers it was shown<br />
that in patients who go into cardiogenic shock after VSR, the prognosis<br />
was uniformly fatal unless they undergo prompt surgery. In<br />
the same study it was found that a higher age correlated with an<br />
adverse outcome. So, early surgery should be considered for every<br />
patient with VSR after AMI in the knowledge that elderly patients<br />
in cardiogenic shock have the worst prognosis [4]. The operation<br />
involves excluding rather than excising the infarcted septum and<br />
ventricular wall. A left ventriculotomy is made through the infarcted<br />
area of muscle and a pericardial patch is sewn over the endocardium<br />
of the left ventricle around the infarcted area of myocardium. The<br />
ventriculotomy is then closed over the pericardial patch.[5]<br />
Patients with VSR are regularly treated with an IABP which improves<br />
survival by augmenting coronary blood flow as well as reducing<br />
left ventricular afterload and wall tension. Indeed, IABP support<br />
results in lower immediate postoperative mortality rates, although<br />
IABP treatment was not found to be associated with improved longterm<br />
survival [6]. In our patient it was decided not to insert an IABP<br />
because of severe atherosclerosis of the femoral arteries.<br />
Alternative insertion sites (e.g. intra-thoracic) were not feasible<br />
because of severe atherosclerosis and elongation of the aorta. The<br />
development of residual or recurrent VSR is reported in up to 28%<br />
of patients who survive surgical repair, and is associated with high<br />
mortality [7].<br />
Nowadays VSR is an extremely rare complication of AMI. VSR<br />
should be considered in all patients who deteriorate rapidly after an<br />
532<br />
neth j crit care • volume 10 • no 5 • october 2006