- Page 1 and 2:
IAEA RADIOISOTOPES AND RADIOPHARMAC
- Page 3 and 4:
YTTRIUM-90 AND RHENIUM-188 RADIOPHA
- Page 5 and 6:
IAEA RADIOISOTOPES AND RADIOPHARMAC
- Page 7 and 8:
FOREWORD The IAEA helps to promote
- Page 9 and 10:
CONTENTS CHAPTER 1. INTRODUCTION ..
- Page 11 and 12:
7.4. Discussion ...................
- Page 13 and 14:
CHAPTER 12. DEVELOPMENT OF 90 Sr/ 9
- Page 15 and 16:
Chapter 1 INTRODUCTION 1.1. BACKGRO
- Page 17 and 18:
devices were designed to improve th
- Page 19 and 20:
etention of the antibody’s target
- Page 21 and 22:
Binding affinity of these derivativ
- Page 23 and 24:
Chapter 2 FUNDAMENTAL CONCEPTS IN R
- Page 25 and 26:
A special characteristic of radioph
- Page 27 and 28:
β particles emitted by 90 Y. Numer
- Page 29 and 30:
2.3.1. Exploitation of certain meta
- Page 31 and 32:
2.3.3. Antibodies The high specific
- Page 33 and 34:
cell cultures, presents one example
- Page 35 and 36:
Another very promising form of radi
- Page 37 and 38:
destructive processes within the jo
- Page 39 and 40:
esearch efforts, since then. Howeve
- Page 41 and 42:
[2.18] HAMILTON, J.G., The metaboli
- Page 43 and 44:
[2.52] GOLDENBERG, D.M., et al., Mu
- Page 45 and 46:
Chapter 3 DEVELOPMENT OF RADIOPHARM
- Page 47 and 48:
FIG. 3.3. Elution efficiencies for
- Page 49 and 50:
FIG. 3.5. Electrodeposition of yttr
- Page 51 and 52:
The results of the experiments of r
- Page 53 and 54:
TABLE 3.3. RESULTS FOR 90 Sr/ 90 Y
- Page 55 and 56:
FIG. 3.11. Elution yield of a 90 Sr
- Page 57 and 58:
According to Fig. 3.12, there is a
- Page 59 and 60:
eagent (Sigma). The mean recovery o
- Page 61 and 62:
FIG. 3.18. Variation of RCP (%) of
- Page 63 and 64:
(20 and 30 min) and volume of 188 R
- Page 65 and 66:
FIG. 3.24. Variation of labelling y
- Page 67 and 68:
(c) Clodronate: 20 mg of clodronate
- Page 69 and 70:
Chapter 4 EVALUATION OF THE 90 Sr/
- Page 71 and 72:
Kamadhenu consists of five main com
- Page 73 and 74:
Quality control of radiolabelling w
- Page 75 and 76:
The electrochemical deposition of 9
- Page 77 and 78:
FIG. 4.5. Typical pattern of the wh
- Page 79 and 80:
FIG. 4.6. Elution profile from the
- Page 81 and 82:
FIG. 4.8. CD20 recognition in Ramos
- Page 83 and 84:
4.5. RECOMMENDATIONS AND FUTURE WOR
- Page 85 and 86:
adioiodine labelled anti-NCAM antib
- Page 87 and 88:
FIG. 5.1. Comparison of biodistribu
- Page 89 and 90:
FIG. 5.3. Three step strategy. Biot
- Page 91 and 92:
TABLE 5.1. THREE STEP PRETARGETING:
- Page 93 and 94:
5.1.4. Therapeutic experiments with
- Page 95 and 96:
5.1.4.4. Conclusion The radioiodine
- Page 97 and 98:
control procedure for detection of
- Page 99 and 100:
6.1.3. Recovery of doped 85/89 Sr 2
- Page 101 and 102:
6.2. PREPARATION AND BIOEVALUATION
- Page 103 and 104:
(a) (b) (c) FIG. 6.5. PD-10 column
- Page 105 and 106:
FIG. 6.7. SDS PAGE pattern of ritux
- Page 107 and 108:
FIG. 6.10. HPLC pattern of pure 90
- Page 109 and 110:
FIG. 6.13. Cell binding studies wit
- Page 111 and 112:
6.2.1.6. Conclusion The procedure f
- Page 113 and 114:
FIG. 6.17. Elution profile of 90 Y
- Page 115 and 116:
form NADH, which, in turn, causes t
- Page 117 and 118:
FIG. 6.19. In vivo distribution pat
- Page 119 and 120:
ACKNOWLEDGEMENTS The authors of thi
- Page 121 and 122:
Chapter 7 DEVELOPMENT OF THERAPEUTI
- Page 123 and 124:
the residual breast tissue becoming
- Page 125 and 126:
7.2.5.2. Automatic procedure The la
- Page 127 and 128:
FIG. 7.2. RCP values of 90 Y r-BHD
- Page 129 and 130:
FIG. 7.3. Pyrogen test shows result
- Page 131 and 132:
to avidin at the 1:4 avidin:r-BHD m
- Page 133 and 134:
[7.9] SU, F.M., GUSTAVSON, L.M., AX
- Page 135 and 136:
8.1. INTRODUCTION In past years, th
- Page 137 and 138:
(a) FIG. 8.1. (a) Schematic illustr
- Page 139 and 140:
Preliminary synthesis of the biotin
- Page 141 and 142:
(a) (b) FIG. 8.4. (a) PEGylated and
- Page 143 and 144:
TABLE 8.2. BIODISTRIBUTION IN RATS
- Page 145 and 146:
A specific binding to avidin was ev
- Page 147 and 148:
[8.13] BOSCHI, A., DUATTI, A., UCCE
- Page 149 and 150:
90 Y solution obtained from a 90 Sr
- Page 151 and 152:
From each fraction, a ~1 g aliquot
- Page 153 and 154:
FIG. 9.2. Relationship between 90 S
- Page 155 and 156:
with 188 Re obtained from the 188 W
- Page 157 and 158:
9.3.2. Labelling of DPA ale DPA ale
- Page 159 and 160:
FIG. 9.8. TLC radiochromatogram of
- Page 161 and 162:
(-) 188 Re(CO) 3 (+) (-) 99m Tc(CO)
- Page 163 and 164:
the HSA solution containing sodium
- Page 165 and 166:
TABLE 9.3. RESULTS OF 99m Tc LABELL
- Page 167 and 168:
9.4.4. Yttrium-90 and 177 Lu labell
- Page 169 and 170:
FIG. 9.14. In vitro stability of 90
- Page 171 and 172:
(a) (b) (c) (d) (e) (f) (g) (h) (i)
- Page 173 and 174:
TABLE 9.8. YTTRIUM-90 CITRATE COLLO
- Page 175 and 176:
(a) (b) FIG. 9.17. Grain size distr
- Page 177 and 178:
TABLE 9.10. BIODISTRIBUTION AFTER A
- Page 179 and 180:
FIG. 9.19. Structure of DOTA biotin
- Page 181 and 182:
[9.8] WESTERA, G., GADZE, A., HORST
- Page 183 and 184:
Chapter 10 DEVELOPMENT, PREPARATION
- Page 185 and 186:
acidic pH) and the vial heated for
- Page 187 and 188:
10.2.2.1. Materials and methods (a)
- Page 189 and 190:
Using a semiquantitative method, th
- Page 191 and 192:
FIG. 10.3. Whole body, SPECT and ta
- Page 193 and 194:
TABLE 10.4. ORGAN DISTRIBUTION STUD
- Page 195 and 196:
was assessed by measuring the relea
- Page 197 and 198:
—— Biological studies: The whol
- Page 199 and 200:
or liver) (see Fig. 10.7). The resu
- Page 201 and 202:
(b) Results and discussion Healthy
- Page 203 and 204:
and the pellet (particles of HA lab
- Page 205 and 206:
FIG. 10.11. Organ distribution stud
- Page 207 and 208:
The procedure was as follows: —
- Page 209 and 210:
TABLE 10.5. INFLUENCE OF CHEMICAL I
- Page 211 and 212: analysed using ICP OES was within t
- Page 213 and 214: suggested for separation of pure 86
- Page 215 and 216: adionuclidic purity of the 90 Y sol
- Page 217 and 218: FIG. 10.16. (a) Strontium-90/yttriu
- Page 219 and 220: [10.5] DJOKIĆ, D.J., JANKOVIĆ, D.
- Page 221 and 222: Yttrium-90 is one of the radionucli
- Page 223 and 224: (a) (b) (c) The generator was prepa
- Page 225 and 226: —— Development of EPC: The EPC
- Page 227 and 228: FIG. 11.4. Elution curve of 90 Sr e
- Page 229 and 230: 11.4. YTTRIUM-90 MAbs The use of th
- Page 231 and 232: FIG. 11.6. RCP measured using ITLC
- Page 233 and 234: FIG. 11.9. Biodistribution of 90 Y
- Page 235 and 236: 11.5. YTTRIUM-90 EDTMP Bone metasta
- Page 237 and 238: FIG. 11.14. Electrophoresis of 90 Y
- Page 239 and 240: FIG. 11.15. Particle size distribut
- Page 241 and 242: 11.7. CONCLUSION During this CRP, a
- Page 243 and 244: Chapter 12 DEVELOPMENT OF 90 Sr/ 90
- Page 245 and 246: 12.1.1.4. Acid vapour trap and radi
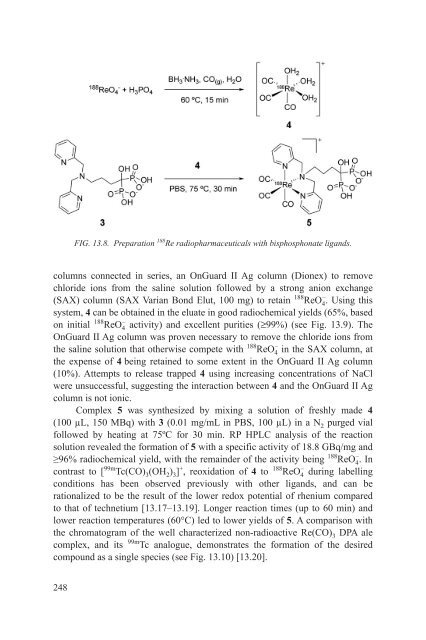
- Page 247 and 248: 12.1.8. Yttrium-90 peptide labellin
- Page 249 and 250: (a) FIG. 12.3. Yttrium-90 elution p
- Page 251 and 252: FIG. 12.6. HPLC of 90 Y DOTATATE. 1
- Page 253 and 254: Chapter 13 BIFUNCTIONAL BISPHOSPHON
- Page 255 and 256: latter requirement [13.10]. When th
- Page 257 and 258: whether the organometallic core sel
- Page 259 and 260: The fate of a targeted imaging prob
- Page 261: FIG. 13.7. SPECT/CT images showing
- Page 265 and 266: FIG. 13.11. Stability study (toward
- Page 267 and 268: Ex vivo biodistribution studies at
- Page 269 and 270: was in the form of either pertechne
- Page 271 and 272: FIG. 13.19. Absolute uptake of 99m
- Page 273 and 274: [13.2] ZHANG, S., GANGAL, G., ULUDA
- Page 275 and 276: [13.31] DE ROSALES, R.T.M., et al.,
- Page 277 and 278: The activity due to 90 Sr should be
- Page 279 and 280: 14.3.1.2. Operation of the 90 Sr/ 9
- Page 281 and 282: antibody were taken, to which 90 Y
- Page 283 and 284: operation, radiopharmaceutical grad
- Page 285 and 286: 14.4.1.3. Operation of the stage II
- Page 287 and 288: A typical EPC pattern for a 90 Y pr
- Page 289 and 290: FIG. 14.10. Decay curve of carrier
- Page 291 and 292: FIG. 14.12. ITLC of 90 Y rituximab.
- Page 293 and 294: FIG. 14.13. Reaction scheme for lab
- Page 295 and 296: TABLE 14.6. STABILITY (%) OF 90 Y D
- Page 297 and 298: FIG. 14.18. Microsphere albumin siz
- Page 299: [14.3] PANDEY, U., DHAMI, P.S., JAG
- Page 302 and 303: naphthalene and 1.2 g of 2,5-diphen
- Page 304 and 305: A-2.2. Materials The method for sep
- Page 306 and 307: out using a Wallac 1411 spectromete
- Page 308 and 309: A-5.3. Procedure The technique was
- Page 310 and 311: Although acyclic chelators offer ma
- Page 312 and 313:
(b) (c) and 90 Y colloids, both ser
- Page 315:
CONTRIBUTORS TO DRAFTING AND REVIEW
- Page 318 and 319:
INDIA Allied Publishers 1 st Floor,
- Page 320:
A key requirement for the effective