Swissmedic Annual Report 2018
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
40 Market access | Licensing<br />
Certificates for medicinal and<br />
transplant products<br />
Companies with establishment licences may request copies<br />
of their licences (certificates) in German, French, English or<br />
Spanish. These certificates give foreign customers or authorities<br />
confirmation in an internationally standardised<br />
format that a valid establishment licence exists. Companies<br />
that export medicinal or transplant products can apply for<br />
confirmation of the current authorisation status in Switzerland<br />
in French, English or Spanish.<br />
Activities<br />
• Ordering certificates for pharmaceutical products using<br />
the online portal proved effective and simplified<br />
checking. The solution will be rolled out for GMP/GDP<br />
certificates during 2019.<br />
• Looking at the multi-year trend, the number of certificates<br />
ordered remained stable, even if there was a slight<br />
fluctuation compared with 2017.<br />
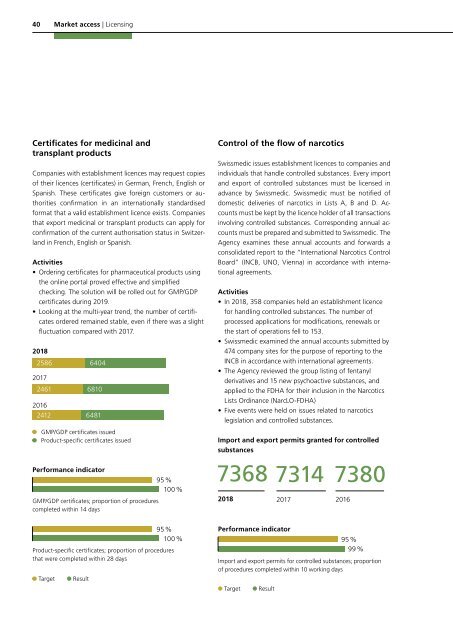
<strong>2018</strong><br />
2586<br />
2017<br />
2461<br />
2016<br />
2412<br />
6404<br />
6810<br />
6481<br />
GMP/GDP certificates issued<br />
Product-specific certificates issued<br />
Performance indicator<br />
GMP/GDP certificates; proportion of procedures<br />
completed within 14 days<br />
95 %<br />
100 %<br />
Control of the flow of narcotics<br />
<strong>Swissmedic</strong> issues establishment licences to companies and<br />
individuals that handle controlled substances. Every import<br />
and export of controlled substances must be licensed in<br />
advance by <strong>Swissmedic</strong>. <strong>Swissmedic</strong> must be notified of<br />
domestic deliveries of narcotics in Lists A, B and D. Accounts<br />
must be kept by the licence holder of all transactions<br />
involving controlled substances. Corresponding annual accounts<br />
must be prepared and submitted to <strong>Swissmedic</strong>. The<br />
Agency examines these annual accounts and forwards a<br />
consolidated report to the “International Narcotics Control<br />
Board” (INCB, UNO, Vienna) in accordance with international<br />
agreements.<br />
Activities<br />
• In <strong>2018</strong>, 358 companies held an establishment licence<br />
for handling controlled substances. The number of<br />
processed applications for modifications, renewals or<br />
the start of operations fell to 153.<br />
• <strong>Swissmedic</strong> examined the annual accounts submitted by<br />
474 company sites for the purpose of reporting to the<br />
INCB in accordance with international agreements.<br />
• The Agency reviewed the group listing of fentanyl<br />
derivatives and 15 new psychoactive substances, and<br />
applied to the FDHA for their inclusion in the Narcotics<br />
Lists Ordinance (NarcLO-FDHA)<br />
• Five events were held on issues related to narcotics<br />
legislation and controlled substances.<br />
Import and export permits granted for controlled<br />
substances<br />
<strong>2018</strong><br />
7314<br />
2017<br />
7380<br />
2016<br />
95 %<br />
100 %<br />
Product-specific certificates; proportion of procedures<br />
that were completed within 28 days<br />
Target<br />
Result<br />
Performance indicator<br />
Import and export permits for controlled substances; proportion<br />
of procedures completed within 10 working days<br />
Target<br />
Result<br />
95 %<br />
99 %