Swissmedic Annual Report 2018
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
74 Stakeholder management<br />
Stakeholder management<br />
Information<br />
<strong>Swissmedic</strong> is quick to provide targeted information on<br />
new findings concerning therapeutic products if there is a<br />
potential risk to health. In addition to safety-relevant information,<br />
new authorisation decisions or major changes to<br />
medicinal product information are of considerable interest.<br />
General enquiries<br />
<strong>Swissmedic</strong> responds to general enquiries submitted by<br />
consumers, patients and specialists on a wide range of subjects<br />
associated with therapeutic products. Generally<br />
speaking, such enquiries are answered within ten days. Enquiries<br />
related to specific applications or cases, and information<br />
and advice provided by <strong>Swissmedic</strong>’s Legal Affairs<br />
staff do not fall within this category.<br />
Activities<br />
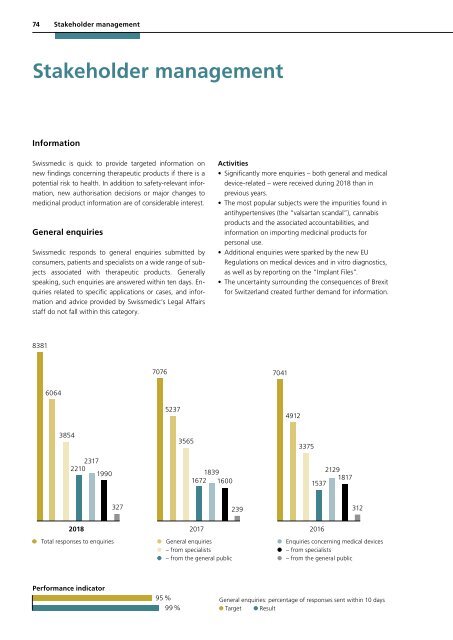
• Significantly more enquiries – both general and medical<br />
device-related – were received during <strong>2018</strong> than in<br />
previous years.<br />
• The most popular subjects were the impurities found in<br />
antihypertensives (the “valsartan scandal”), cannabis<br />
products and the associated accountabilities, and<br />
information on importing medicinal products for<br />
personal use.<br />
• Additional enquiries were sparked by the new EU<br />
Regulations on medical devices and in vitro diagnostics,<br />
as well as by reporting on the “Implant Files”.<br />
• The uncertainty surrounding the consequences of Brexit<br />
for Switzerland created further demand for information.<br />
8381<br />
7076<br />
7041<br />
6064<br />
5237<br />
4912<br />
3854<br />
2317<br />
2210<br />
1990<br />
3565<br />
1839<br />
1672 1600<br />
3375<br />
2129<br />
1817<br />
1537<br />
327<br />
239<br />
312<br />
<strong>2018</strong><br />
2017<br />
Total responses to enquiries General enquiries<br />
– from specialists<br />
– from the general public<br />
2016<br />
Enquiries concerning medical devices<br />
– from specialists<br />
– from the general public<br />
Performance indicator<br />
95 %<br />
99 %<br />
General enquiries: percentage of responses sent within 10 days<br />
Target Result