Swissmedic Annual Report 2018
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
44 Market access l Inspections<br />
GCP and GVP inspections<br />
<strong>Swissmedic</strong> carries out random inspections of clinical trials<br />
of medicinal products in Switzerland. Good Clinical Practice<br />
(GCP) inspections focus on whether the safety and personal<br />
rights of trial participants are guaranteed. They also verify<br />
whether the trials are being conducted in accordance with<br />
the scientific criteria for quality and integrity. Inspections<br />
may examine circumstances relating to one or more clinical<br />
trials. They can focus both on the way trial centres are conducting<br />
a trial (trial centre inspection) and on the way pharmaceutical<br />
companies, contract research organisations<br />
(CROs), pharmacies and research organisations or units are<br />
managing it.<br />
After it has approved a human medicinal product in Switzerland,<br />
<strong>Swissmedic</strong> conducts pharmacovigilance inspections<br />
of authorisation holders (pharmaceutical companies),<br />
as well as of CROs and organisations contracted by authorisation<br />
holders to carry out pharmacovigilance-related activities<br />
on their behalf. These inspections assess whether<br />
the pharmacovigilance processes operated by the companies,<br />
CROs or organisations being inspected comply with<br />
applicable national laws, international Good Vigilance Practice<br />
(GVP) directives and <strong>Swissmedic</strong> requirements.<br />
Activities<br />
• In <strong>2018</strong>, <strong>Swissmedic</strong> carried out 22 GCP inspections of<br />
clinical trials involving medicinal products authorised in<br />
Switzerland, including one trial in Germany and one in<br />
the UK.<br />
• <strong>Swissmedic</strong> also carried out 12 GVP inspections in<br />
Switzerland.<br />
• Within the framework of the Geneva-based PIC/S<br />
(Pharmaceutical Inspection Co-operation Scheme)<br />
convention, <strong>Swissmedic</strong> participated in two GCP<br />
inspection programmes (in Norway and Germany) and<br />
one GVP inspection programme (in Lithuania). One of<br />
the 12 GVP inspections conducted in Switzerland was<br />
also part of the PIC/S programme.<br />
• <strong>Swissmedic</strong>’s GCP/GVP inspectors participated in the<br />
EMA’s Inspectors Working Groups once again in <strong>2018</strong>.<br />
• One GCP inspection was carried out in the transplant<br />
products sector during <strong>2018</strong>.<br />
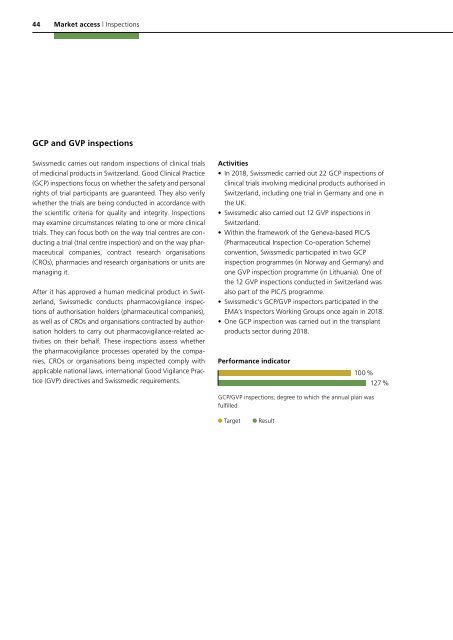
Performance indicator<br />
100 %<br />
127 %<br />
GCP/GVP inspections; degree to which the annual plan was<br />
fulfilled<br />
Target<br />
Result