Swissmedic Annual Report 2018
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Market access | Medical devices<br />
61<br />
Placing on the market<br />
Manufacturers of medical devices that entail an elevated<br />
level of risk must have the conformity assessment of their<br />
products carried out by an officially accredited notified<br />
body. Certain medical devices are subject to mandatory notification.<br />
<strong>Swissmedic</strong> receives the notifications for such<br />
products, checks them at random to ensure the products<br />
have been correctly classified, issues instructions to make<br />
corrections where necessary, and records the notifications<br />
in the European EUDAMED database.<br />
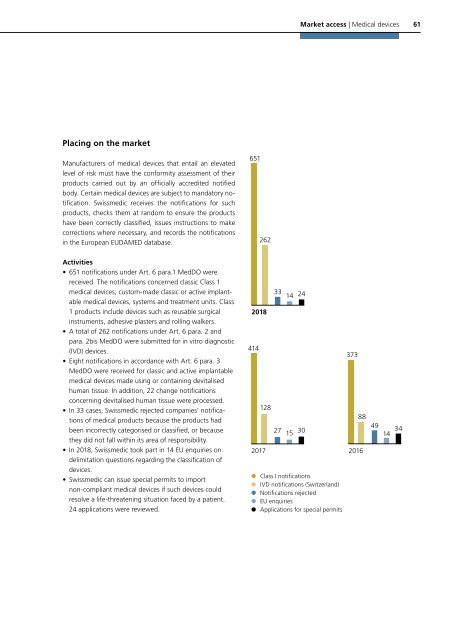
651<br />
262<br />
Activities<br />
• 651 notifications under Art. 6 para.1 MedDO were<br />
received. The notifications concerned classic Class 1<br />
medical devices, custom-made classic or active implantable<br />
medical devices, systems and treatment units. Class<br />
1 products include devices such as reusable surgical<br />
instruments, adhesive plasters and rolling walkers.<br />
• A total of 262 notifications under Art. 6 para. 2 and<br />
para. 2bis MedDO were submitted for in vitro diagnostic<br />
(IVD) devices.<br />
• Eight notifications in accordance with Art. 6 para. 3<br />
MedDO were received for classic and active implantable<br />
medical devices made using or containing devitalised<br />
human tissue. In addition, 22 change notifications<br />
concerning devitalised human tissue were processed.<br />
• In 33 cases, <strong>Swissmedic</strong> rejected companies’ notifications<br />
of medical products because the products had<br />
been incorrectly categorised or classified, or because<br />
they did not fall within its area of responsibility.<br />
• In <strong>2018</strong>, <strong>Swissmedic</strong> took part in 14 EU enquiries on<br />
delimitation questions regarding the classification of<br />
devices.<br />
• <strong>Swissmedic</strong> can issue special permits to import<br />
non-compliant medical devices if such devices could<br />
resolve a life-threatening situation faced by a patient.<br />
24 applications were reviewed.<br />
<strong>2018</strong><br />
414<br />
128<br />
2017<br />
33 14 24<br />
27 15 30<br />
Class I notifications<br />
IVD notifications (Switzerland)<br />
Notifications rejected<br />
EU enquiries<br />
Applications for special permits<br />
373<br />
88<br />
2016<br />
49<br />
14 34