Swissmedic Annual Report 2018
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
56 Market access | Medicinal products<br />
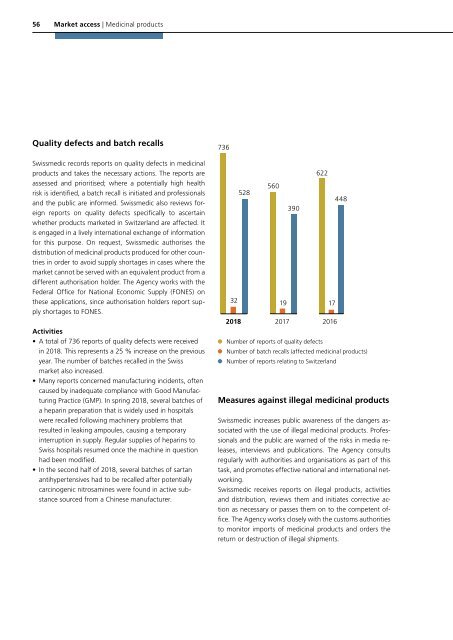
Quality defects and batch recalls<br />
<strong>Swissmedic</strong> records reports on quality defects in medicinal<br />
products and takes the necessary actions. The reports are<br />
assessed and prioritised; where a potentially high health<br />
risk is identified, a batch recall is initiated and professionals<br />
and the public are informed. <strong>Swissmedic</strong> also reviews foreign<br />
reports on quality defects specifically to ascertain<br />
whether products marketed in Switzerland are affected. It<br />
is engaged in a lively international exchange of information<br />
for this purpose. On request, <strong>Swissmedic</strong> authorises the<br />
distribution of medicinal products produced for other countries<br />
in order to avoid supply shortages in cases where the<br />
market cannot be served with an equivalent product from a<br />
different authorisation holder. The Agency works with the<br />
Federal Office for National Economic Supply (FONES) on<br />
these applications, since authorisation holders report supply<br />
shortages to FONES.<br />
Activities<br />
• A total of 736 reports of quality defects were received<br />
in <strong>2018</strong>. This represents a 25 % increase on the previous<br />
year. The number of batches recalled in the Swiss<br />
market also increased.<br />
• Many reports concerned manufacturing incidents, often<br />
caused by inadequate compliance with Good Manufacturing<br />
Practice (GMP). In spring <strong>2018</strong>, several batches of<br />
a heparin preparation that is widely used in hospitals<br />
were recalled following machinery problems that<br />
resulted in leaking ampoules, causing a temporary<br />
interruption in supply. Regular supplies of heparins to<br />
Swiss hospitals resumed once the machine in question<br />
had been modified.<br />
• In the second half of <strong>2018</strong>, several batches of sartan<br />
antihypertensives had to be recalled after potentially<br />
carcinogenic nitrosamines were found in active substance<br />
sourced from a Chinese manufacturer.<br />
736<br />
32<br />
528<br />
560<br />
19<br />
390<br />
622<br />
Measures against illegal medicinal products<br />
<strong>Swissmedic</strong> increases public awareness of the dangers associated<br />
with the use of illegal medicinal products. Professionals<br />
and the public are warned of the risks in media releases,<br />
interviews and publications. The Agency consults<br />
regularly with authorities and organisations as part of this<br />
task, and promotes effective national and international networking.<br />
<strong>Swissmedic</strong> receives reports on illegal products, activities<br />
and distribution, reviews them and initiates corrective action<br />
as necessary or passes them on to the competent office.<br />
The Agency works closely with the customs authorities<br />
to monitor imports of medicinal products and orders the<br />
return or destruction of illegal shipments.<br />
17<br />
<strong>2018</strong> 2017 2016<br />
Number of reports of quality defects<br />
448<br />
Number of batch recalls (affected medicinal products)<br />
Number of reports relating to Switzerland