Swissmedic Annual Report 2018
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
2018 Annual Report and annual financial statements of the Swiss Agency for Therapeutic Products (Swissmedic)
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Market access | Licensing<br />
41<br />
Clinical trials with medicinal products<br />
Clinical trials are used to systematically gather information<br />
on medicinal products when used in humans. <strong>Swissmedic</strong><br />
verifies whether the quality and safety of the test product is<br />
guaranteed. Clinical trials may only be carried out in Switzerland<br />
if they have been approved by an Ethics Committee<br />
and by <strong>Swissmedic</strong>.<br />
Activities<br />
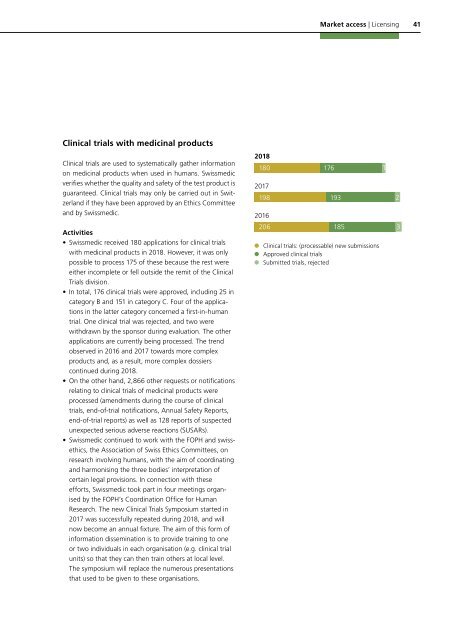
• <strong>Swissmedic</strong> received 180 applications for clinical trials<br />
with medicinal products in <strong>2018</strong>. However, it was only<br />
possible to process 175 of these because the rest were<br />
either incomplete or fell outside the remit of the Clinical<br />
Trials division.<br />
• In total, 176 clinical trials were approved, including 25 in<br />
category B and 151 in category C. Four of the applications<br />
in the latter category concerned a first-in-human<br />
trial. One clinical trial was rejected, and two were<br />
withdrawn by the sponsor during evaluation. The other<br />
applications are currently being processed. The trend<br />
observed in 2016 and 2017 towards more complex<br />
products and, as a result, more complex dossiers<br />
continued during <strong>2018</strong>.<br />
• On the other hand, 2,866 other requests or notifications<br />
relating to clinical trials of medicinal products were<br />
processed (amendments during the course of clinical<br />
trials, end-of-trial notifications, <strong>Annual</strong> Safety <strong>Report</strong>s,<br />
end-of-trial reports) as well as 128 reports of suspected<br />
unexpected serious adverse reactions (SUSARs).<br />
• <strong>Swissmedic</strong> continued to work with the FOPH and swissethics,<br />
the Association of Swiss Ethics Committees, on<br />
research involving humans, with the aim of coordinating<br />
and harmonising the three bodies’ interpretation of<br />
certain legal provisions. In connection with these<br />
efforts, <strong>Swissmedic</strong> took part in four meetings organised<br />
by the FOPH’s Coordination Office for Human<br />
Research. The new Clinical Trials Symposium started in<br />
2017 was successfully repeated during <strong>2018</strong>, and will<br />
now become an annual fixture. The aim of this form of<br />
information dissemination is to provide training to one<br />
or two individuals in each organisation (e.g. clinical trial<br />
units) so that they can then train others at local level.<br />
The symposium will replace the numerous presentations<br />
that used to be given to these organisations.<br />
<strong>2018</strong><br />
180 176<br />
2017<br />
198 193<br />
2016<br />
206 185 3<br />
Clinical trials: (processable) new submissions<br />
Approved clinical trials<br />
Submitted trials, rejected<br />
2