Gastroenterology Today Autumn 2020
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Volume 30 No. 3<br />
<strong>Autumn</strong> <strong>2020</strong><br />
<strong>Gastroenterology</strong> <strong>Today</strong><br />
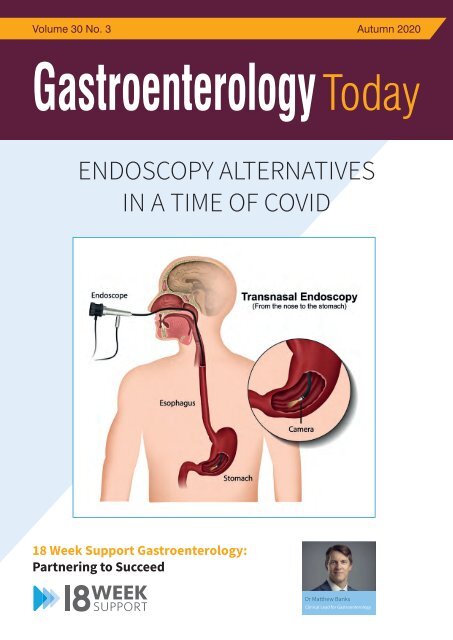
ENDOSCOPY ALTERNATIVES<br />
IN A TIME OF COVID<br />
What approach has 18 Week Support<br />
taken with regards to building an<br />
expert insourcing team?<br />
Matthew’s Perspective:<br />
Dr Matthew Banks is the Clinical Director for 18 Week Support <strong>Gastroenterology</strong>. He believes it starts with recruiting the<br />
best clinicians. ‘At 18 Week Support we set the bar very high. We only recruit clinicians whose JAG performance data is well<br />
above the national standards. In addition, we monitor each clinician’s KPIs while they work with 18 WS. While the JAG data<br />
is an excellent quality indicator, we now want to go a step beyond that and monitor the Non-Technical skills (NTS) of each<br />
clinician as well. We now know that NTS plays an important role in safe and effective team performance. Therefore, in our<br />
quest to develop excellent teams who deliver a world-class service, we must focus on NTS’.<br />
Tammy and Lisa’s Perspective:<br />
Tammy Kingstree is Lead Nurse for Endoscopy.<br />
‘It is extremely important that there are good working relationships within the team. This starts with strong leadership from<br />
our senior nurse coordinators who are trained to manage the patient pathway, manage a team of staff they may not know<br />
and to deal effectively with any issues which may arise on the day’.<br />
Lisa Phillips is Lead Nurse for Endoscopy.<br />
‘The team objectives are clear. Excellent patient experience and good patient outcomes. Because the objectives are clear,<br />
team cohesion and focus are exceptionally good. It therefore shouldn’t matter that we are in an unfamiliar endoscopy unit,<br />
the service should be seamless. If it isn’t, we do not stop until we get it right.<br />
If you have an excellent NHS record and want to help clear NHS waiting list backlogs, reduce RTT waiting times and provide<br />
18 Week Support <strong>Gastroenterology</strong>:<br />
Partnering to Succeed<br />
high-quality patient care, get in touch by calling on 020 3892 6162 or email Gastro.Recruitment@18weeksupport.com<br />
Dr Matthew Banks<br />
Clinical Lead for <strong>Gastroenterology</strong>
H<br />
Find out more at www.alphalabs.co.uk<br />
Simple Solutions to Support<br />
Clinical Decision Making<br />
in <strong>Gastroenterology</strong><br />
Calprotectin<br />
Testing<br />
Make more informed clinical decisions<br />
without waiting for lab results.<br />
The future for IBD Care:<br />
■ IBDoc ® Home Tests<br />
Supporting remote patient<br />
monitoring and virtual clinics<br />
■ Quantum Blue ® for Point of Care<br />
Helps triage patients in clinic<br />
giving results in a rapid<br />
time frame (15 minutes)<br />
Faecal Faecal<br />
Immunochemical<br />
Testing Testing<br />
Triage Triage patients within within the the colorectal<br />
cancer colorectal pathway cancer pathway to better<br />
manage colonoscopy resources.<br />
■ Complete customised FIT<br />
■‘Patient Complete Packs’ customised FIT<br />
‘Patient Packs’<br />
■ Include everything the patient<br />
■requires Include to everything take their the sample patient<br />
safely requires at home to take and their return sample it to<br />
the safely laboratory. at home and return it to<br />
the laboratory.<br />
For more information, to discuss your requirements or organise<br />
an evaluation please contact: digestivedx@alphalabs.co.uk<br />
Supplied by<br />
g<br />
999123<br />
2021.12.31<br />
Name<br />
80101274<br />
Date of Sample<br />
NAME<br />
Mr<br />
Ms<br />
M / F<br />
Date of Birth (DD/MM/YYYY)<br />
HOW to Pl<br />
/ /<br />
1. Preparation<br />
Date of Sampling (DD/MM/YYYY)<br />
/ /<br />
l<br />
Write<br />
your NAME and Date of Birth on the<br />
Green Plastic Bag and Device.<br />
Carefully and slowly twist and pull out the<br />
Stick Part from Main BODY.<br />
T: +44 (0)23 8048 3000<br />
E: sales@alphalabs.co.uk<br />
W: www.alphalabs.co.uk
CONTENTS<br />
CONTENTS<br />
5 EDITORS COMMENT<br />
6 FEATURE Transplantation during the COVID-19 pandemic:<br />
Matthew’s Perspective:<br />
nothing noble is accomplished without danger<br />
12 FEATURE Rethinking how we treat constipation in the UK<br />
16 NEWS<br />
22 COMPANY NEWS<br />
COVER STORY<br />
ENDOSCOPY ALTERNATIVES IN A TIME OF COVID – Innovative thinking<br />
and different ways of working to clear NHS Trusts Waiting Lists<br />
<strong>Gastroenterology</strong> <strong>Today</strong><br />
What approach has 18 Week Support<br />
taken with regards to building an<br />
expert insourcing team?<br />
This issue edited by:<br />
Dr Andrew Poullis<br />
c/o Media Publishing Company<br />
Media House<br />
48 High Street<br />
Dr Matthew Banks is the Clinical Director for 18 Week Support <strong>Gastroenterology</strong>. SWANLEY, He believes Kent it starts BR8 with recruiting 8BQ the<br />
best clinicians. ‘At 18 Week Support we set the bar very high. We only recruit clinicians whose JAG performance data is well<br />
above the national standards. In addition, we monitor each clinician’s KPIs while they work with 18 WS. While the JAG data<br />
is an excellent quality indicator, we now want to go a step beyond that and ADVERTISING monitor the Non-Technical & CIRCULATION:<br />
skills (NTS) of each<br />
clinician as well. We now know that NTS plays an important role in safe and Media effective Publishing team performance. Company<br />
Therefore, in our<br />
quest to develop excellent teams who deliver a world-class service, we must Media focus on House, NTS’. 48 High Street<br />
SWANLEY, Kent, BR8 8BQ<br />
Tammy and Lisa’s Perspective:<br />
Tammy Kingstree is Lead Nurse for Endoscopy.<br />
Tel: 01322 660434 Fax: 01322 666539<br />
‘It is extremely important that there are good working relationships within E: the info@mediapublishingcompany.com<br />
team. This starts with strong leadership from<br />
our senior nurse coordinators who are trained to manage the patient pathway, manage a team of staff they may not know<br />
www.MediaPublishingCompany.com<br />
and to deal effectively with any issues which may arise on the day’.<br />
Lisa Phillips is Lead Nurse for Endoscopy.<br />
PUBLISHING DATES:<br />
‘The team objectives are clear. Excellent patient experience and good patient March, outcomes. June, Because September the objectives and are clear, December.<br />
team cohesion and focus are exceptionally good. It therefore shouldn’t matter that we are in an unfamiliar endoscopy unit,<br />
the service should be seamless. If it isn’t, we do not stop until we get it right.<br />
COPYRIGHT:<br />
If you have an excellent NHS record and want to help clear NHS waiting list Media backlogs, Publishing reduce RTT waiting Company<br />
times and provide<br />
high-quality patient care, get in touch by calling on 020 3892 6162 or email Gastro.Recruitment@18weeksupport.com<br />
Media House<br />
48 High Street<br />
SWANLEY, Kent, BR8 8BQ<br />
PUBLISHERS STATEMENT:<br />
The views and opinions expressed in<br />
this issue are not necessarily those of<br />
the Publisher, the Editors or Media<br />
Publishing Company.<br />
For the next 12 months and probably longer, the impact of COVID on diagnostic<br />
pathways will have far reaching effects on waiting lists and time to diagnosis.<br />
Diseases of the gastrointestinal tract can have a devastating impact on health<br />
so rapid diagnosis and management of these diseases is vital to ensure positive<br />
outcomes for patients.<br />
Exploring alternative diagnostic technologies should be a vital component for the<br />
NHS in assessing new strategies to cope with this significant increase in demand,<br />
especially where they can deliver results quickly, safely and cost-effectively.<br />
Endoscopy has not been immune from technological innovation, for example<br />
FIT, Cytosponge and Pillcam. Each of these offers some cost, accuracy or other<br />
benefits to Trusts at this time, and we propose to review these in subsequent<br />
editions. However, in this edition we start with Transnasal endoscopy as our first<br />
alternative technology to be explored.<br />
We know it can be deployed safely and easily in outpatient settings, and at this time<br />
any keeping patients and surgical teams separate from hospital red zones is an<br />
important advantage at this current time.<br />
Next Issue Winter <strong>2020</strong><br />
Subscription Information – <strong>Autumn</strong> <strong>2020</strong><br />
<strong>Gastroenterology</strong> <strong>Today</strong> is a quarterly<br />
publication currently sent free of charge to<br />
all senior qualified Gastroenterologists in<br />
the United Kingdom. It is also available<br />
by subscription to other interested individuals<br />
and institutions.<br />
UK:<br />
Other medical staff - £18.00 inc. postage<br />
Non-medical Individuals - £24.00 inc. postage<br />
Institutions<br />
Libraries<br />
Commercial Organisations - £48.00 inc. postage<br />
Rest of the World:<br />
Individuals - £48.00 inc. postage<br />
Institutions<br />
Libraries<br />
Commercial Organisations - £72.00 inc. postage<br />
We are also able to process your<br />
subscriptions via most major credit<br />
cards. Please ask for details.<br />
Cheques should be made<br />
payable to MEDIA PUBLISHING.<br />
Designed in the UK by me&you creative<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
3
EDITORS COMMENT<br />
EDITORS COMMENT<br />
Covid Collateral<br />
The health and economic impacts of Covid are increasingly being understood. In addition<br />
to the obvious direct costs the collateral damage is starting to become evident.<br />
“The<br />
longer term<br />
consequences<br />
of this<br />
pandemic<br />
may not yet<br />
be evident.”<br />
Some of this collateral damage, with delays in diagnosis and treatments, is obvious and<br />
hopefully relatively short lived. The longer term consequences of this pandemic may not yet<br />
be evident.<br />
The unprecedented cessation of diagnostic endoscopy has led to the build up of enormous<br />
waiting lists. Trusts are struggling to tackle these for new patients but of equal concern is<br />
the collateral damage “2nd wave” of delays to surveillance patients. Endoscopic follow up<br />
of Barrett’s, IBD, colorectal cancer and colonic polyps constitutes a large volume of work<br />
within luminal gastroenterology. The gains made in endoscopy quality are at risk if the false<br />
solution of overbooking lists (as has been suggested) to deal with this waiting list issue are<br />
forced through. A high quality service needs time, correctly trained staff and the appropriate<br />
physical space to deliver the service - this is just as true of radiology and out-patient<br />
services as it is to endoscopy.<br />
While the NHS doesn’t need re-building it certainly needs quite a bit of maintenance -<br />
without this the legacy of Covid is likely to be more protracted than the duration of the<br />
pandemic.<br />
A Poullis<br />
ScheBo_Gastro<strong>Today</strong>_Aug_<strong>2020</strong> 14/08/<strong>2020</strong> St George’s Hospital<br />
ScheBo_Gastro<strong>Today</strong>_Aug_<strong>2020</strong> Multi-Ad_Address_Change<br />
®<br />
ScheBo • SARS-CoV-2 Quick<br />
15 minute blood test for IgM<br />
(current infection) and IgG<br />
(past infection) antibodies<br />
<br />
NEW!<br />
EMAIL<br />
COVID@SCHEBO.CO.UK<br />
FOR DETAILS!<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
Or for further information please contact:<br />
®<br />
Ivor Smith, ScheBo • Biotech UK Ltd, PO Box 9459,<br />
Lyme Regis, DT6 9FL<br />
Tel: 01256 477259 email: i.smith@schebo.co.uk<br />
www.schebo.co.uk<br />
5
FEATURE<br />
TRANSPLANTATION DURING THE<br />
COVID-19 PANDEMIC: NOTHING NOBLE<br />
IS ACCOMPLISHED WITHOUT DANGER<br />
Gabriele Spoletini 1* , Giuseppe Bianco 1 , Dario Graceffa 2 and Quirino Lai 3<br />
Spoletini et al. BMC <strong>Gastroenterology</strong> (<strong>2020</strong>) 20:259 https://doi.org/10.1186/s12876-020-01401-0 © The Author(s). <strong>2020</strong><br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
6<br />
Abstract<br />
The global health crisis due to the fast spread of coronavirus disease<br />
(COVID-19) has caused major disruption in all aspects of healthcare.<br />
Transplantation is one of the most affected sectors, as it relies on a<br />
variety of services that have been drastically occupied to treat patients<br />
affected by COVID-19. With this report from two transplant centers<br />
in Italy, we aim to reflect on resource organization, organ allocation,<br />
virus testing and transplant service provision during the course of<br />
the pandemic and to provide actionable information highlighting<br />
advantages and drawbacks. To what extent can we preserve the noble<br />
purpose of transplantation in times of increased danger? Strategies to<br />
minimize risk exposure to the transplant population and health- workers<br />
include systematic virus screening, protection devices, social distancing<br />
and reduction of patients visits to the transplant center. While resources<br />
for the transplant activity are inevitably reduced, new dilemmas arise to<br />
the transplant community: further optimization of time constraints during<br />
organ retrievals and implantation, less organs and blood products<br />
donated, limited space in the intensive care unit and the duty to<br />
maintain safety and outcomes.<br />
Keywords:<br />
Coronavirus, Transplantation, Organ donation, SARS-CoV-2, COVID-19,<br />
Virus tests.<br />
Background<br />
Since December 2019, the fast spread of the novel Severe Acute<br />
Respiratory Syndrome CoronaVirus-2 (SARS-CoV-2) causing a severe<br />
acute respiratory disease (COVID-19), has determined a healthcare<br />
crisis in a growing number of countries. To date, USA, Spain and Italy<br />
have reported the highest number of patients affected, and COVID-19<br />
has been categorized as a global pandemic [1]. Disruptions in<br />
almost all aspects of health care provision have been observed, and<br />
health systems are trying to continue offering essential services while<br />
suspending those that can be postponed.<br />
Transplant services can be categorized depending on their lifesaving<br />
nature. Heart, lung and liver transplants are urgent lifesaving operations<br />
in a proportion of wait-listed patients. In particular, those with chronic<br />
end-stage organ disease who develop deterioration of their baseline<br />
condition, and those who suffer from sudden end-stage failure of a<br />
given organ.<br />
*<br />
Correspondence: gabriele.spoletini@policlinicogemelli.it<br />
1<br />
General Surgery and Liver Transplantation, Fondazione Policlinico<br />
Universitario A. Gemelli IRCCS, Largo Agostino Gemelli 8, 00168 Rome, Italy<br />
While it seems obvious that lifesaving transplant activity should not<br />
be stopped, it is not clear whether nonlifesaving transplants should<br />
be delayed past the most critical phase of the emergency. In fact,<br />
prolonging the time spent on the waiting list can translate into waiting list<br />
drop-out due to disease progression or overcoming contra-indications.<br />
On May 3, <strong>2020</strong>, Italy is the third most affected country worldwide<br />
and has registered the second highest number of COVID-19-related<br />
deaths so far. The Italian National Authority for Transplantation released<br />
guidance on donor and recipient testing for SARS-CoV-2 [2, 3]. Testing<br />
via naso-pharyngeal swab (NPS) or bronchoalveolar lavage and, if<br />
positive, measurement of viral load on blood sample are recommended<br />
in all donors from high incidence regions. SARS-CoV-2 positive potential<br />
deceased donors are to be discarded and living donors postponed.<br />
NPS is compulsory before transplantation for all potential recipients who<br />
are symptomatic or with a history of contact with a COVID-19 positive<br />
patient, and discretional for asymptomatic recipients in whom history of<br />
contact with COVID-19 positive patient can be reasonably ruled out.<br />
Implications of the spread of COVID-19 for the transplant community<br />
are innumerable, and the unprecedented nature of the pandemic has<br />
left physicians without guidance in many of their management choices.<br />
Balancing resource constraints, patient safety and life-saving organs<br />
demand is difficult during COVID-19 pandemic. With the present report<br />
we aim to reflect on the open challenges for the transplant community.<br />
A summary of actions to be undertaken is summarized, reflecting on<br />
advantages and dangers related to each (Table 1).<br />
Screening and risk exposure for transplant staff<br />
Since the beginning of the pandemic, health-workers screening<br />
has been advocated as an essential tool for: 1) protecting patients<br />
from staff-mediated transmission and 2) protecting health-workers<br />
allowing prompt treatment. In the setting of transplantation, the first is<br />
of paramount importance, being the immunosuppressed population<br />
more vulnerable to infections. As of February 11, <strong>2020</strong>, out of 44,672<br />
confirmed COVID-19 cases in Mainland China, 1716 (3.8%) cases were<br />
health-workers [4]. To date, 21,338 health-workers have tested positive<br />
for SARS-CoV-2 in Italy and 154 doctors (including retired ones) and<br />
40 nurses lost their lives after being infected [5]. Shortage of personal<br />
protection devices and work overload have contributed to increase<br />
the rate of contagion within health-workers. Hosts of SARS-CoV-2 may<br />
transmit the virus while they are asymptomatic or during the incubation<br />
period, a mechanism that creates a vicious circle of in-hospital disease<br />
spread to patients and staff. Testing all the transplant staff (or, at least,<br />
those who come into contact with transplanted patients) could mitigate
FEATURE<br />
Table 1 Summary of issues and actions to be undertaken to mitigate the risks for the transplant population and staff related to<br />
COVID-19<br />
Issues and actions Advantage Disadvantage<br />
Screening and risk exposure for transplant staff<br />
Extensive screening of transplant staff<br />
Travels reduction – regional organs shipping systems<br />
Timing and logistics of transplantation<br />
Screening of waitlisted patients<br />
Healthcare workers safety<br />
Breaking the vicious circle of in-hospital virus<br />
transmission<br />
Reduction of contagion to other hospitals<br />
from travelling retrieval surgeons<br />
Thorough information regarding patients<br />
awaiting transplants<br />
Increased costs<br />
More staff quarantined<br />
Need to develop a graft exchange<br />
system if not in place yet<br />
Costs<br />
Logistics of testing for patients<br />
currently out-of-hospital<br />
Recipients testing at the time of transplant offer Lower costs compared to previous action Delays before transplant start<br />
Possible cancellation of recipient’s<br />
transplant<br />
Back-up recipient in hospital<br />
Use of machine perfusions to fast-track organ retrieval from<br />
unstable donors (applicable only to donors with low-risk<br />
COVID-19 history)<br />
Teleclinics for follow-up of transplant recipients<br />
Transplant benefit<br />
Revisiting local policies of access to transplantation based<br />
on hospital resources availability<br />
• Privileging “utility” (recipients with expected better<br />
outcomes)<br />
• Privileging “urgency” (recipients with the highest need)<br />
Prompt replacement if first candidate tests<br />
positive<br />
Extended preservation time<br />
Higher organs yield<br />
Avoiding access to hospital out-patient clinics<br />
- decreased exposure to infection<br />
Realistic approach to resource allocation<br />
between COVID and non-COVID diseases<br />
• Less resource consumption (faster ICU<br />
turnaround, less blood transfusions, etc.)<br />
• Treating the sickest patients only and utilize<br />
resources for those in desperate need of<br />
transplantation<br />
More complex logistics<br />
Anxiety and potential frustration for<br />
most back-up patients<br />
Increased logistics costs.<br />
Increased costs<br />
Aborted procedures if COVID-19 tests<br />
return positive<br />
Increased risk of missing potentially<br />
relevant yet subclinical health<br />
problems<br />
Further stretching healthcare<br />
resources with risk of system collapse<br />
• Missing the sickest patients;<br />
increased mortality without treatment<br />
• Uncertainty regarding mortality<br />
effect at the “bottom” of the<br />
transplant waiting list<br />
the risk of in-hospital transmission at the price of increased costs and<br />
workload for already under-pressure health systems. In Italy, during the<br />
fast-growing spread of COVID-19 in March, the lack of tests did not<br />
allow to adopt such an extensive screening policy.<br />
countries where social distancing measures have been in place for as<br />
long as the median virus incubation time, have the opportunity to rule<br />
out possible false negative tests from recipients who have complied with<br />
the social restriction policy [6, 7].<br />
In addition, transplant teams are at higher risk of contagion as they might<br />
travel to high incidence areas when retrieving organs for transplantation.<br />
Some countries do not have a centralized organ retrieval system and<br />
transplant teams travel outside their regions to procure organs they<br />
will implant. A “travelling organs” policy such as in the National Organ<br />
Retrieval System in the United Kingdom or Euro-transplant in central<br />
Europe help avoid transplant teams travelling from low to high incidence<br />
regions and contain the spread within medical staff. In our region, liver<br />
transplant centers based in Rome share an organ procurement scheme<br />
to retrieve and ship organs to other centers in Italy. Most regions in Italy<br />
have implemented a regional organ sharing system which, during the<br />
COVID-19 pandemic, has been increasingly utilized.<br />
Timing and logistics of transplantation<br />
Due to the relevant number of false negative viral tests, there is a<br />
consistent risk of transplanting recipients who are either asymptomatic<br />
or in the incubation phase. This mandates caution and candidates<br />
for transplantation are delayed if their condition allows to. However,<br />
Success of transplantation relies on optimization of time constraints. The<br />
additional time required for COVID-19 testing of donors and recipients<br />
may delay organ procurement and lower the utilization rate especially<br />
of hemodynamically unstable donors that normally require fast-track<br />
management to minimize organs damage. Machine perfusion for organ<br />
preservation is expanding in almost all solid organs transplantation,<br />
allowing extend preservation time in liver, kidney, lung and heart<br />
transplantation [8, 9]. Machine perfusion could come into help when<br />
organs need to be retrieved quickly and preserved while virus tests<br />
are processed, in particular in unstable donors with low-risk history for<br />
COVID-19.<br />
In an effort to minimize the possibility of delays which cause prolongation<br />
of cold ischemia time, back-up transplant candidates have been called<br />
in as a routine policy by several transplant centers when issues with the<br />
first-choice candidate are anticipated. Implementing such policy during<br />
the COVID-19 outbreak could offer the possibility to quickly replace the<br />
first candidate if they turn out to be SARS-CoV-2 positive.<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
7
FEATURE<br />
Table 2 Diagnostic tests available in Italy to detect SARS-CoV-2 infection<br />
Method<br />
Real time reverse<br />
transcriptionpolymerase<br />
chain<br />
reaction<br />
Direct amplification<br />
real-time reverse<br />
transcriptionpolymerase<br />
chain reaction.<br />
Diasorin<br />
Simplexa<br />
Solid phase immunochromatographic<br />
assay for the detection<br />
of IgG and IgM<br />
antibodies to SARS-<br />
CoV-2.<br />
Type of specimen<br />
required<br />
Respiratory and<br />
non-respiratory<br />
tract specimens<br />
Nasopharyngeal<br />
swabs<br />
Whole blood,<br />
serum or plasma<br />
Time required for assay Advantages<br />
5–8 h Gold standard for the etiological<br />
diagnosis; high sensitivity and<br />
specificity; high safety<br />
1 h High sensitivity and specificity;<br />
simple protocol with all in one<br />
reagent; rapid response; high<br />
safety; suitable for decentralized<br />
point-of-care<br />
5–15 min No equipment needed; rapid<br />
response; suitable for decentralized<br />
point-of-care; good sensitivity and<br />
specificity; suitable for identifying<br />
asymptomatic patients and for<br />
screening<br />
Limits<br />
Complex protocol; overcoming of the<br />
throughput capacities of the laboratories<br />
with diagnostic delays; not suitable for<br />
decentralized point-of-care<br />
For emergency use authorization only;<br />
Limited literature data; Limited to<br />
laboratories certified to perform high<br />
complexity tests<br />
Not recommended as first line test for<br />
the diagnosis of acute viral infection;<br />
prone to ‘cross reactivity’; few reports<br />
about serological assay in detection of<br />
SARS-CoV-2; uncertain timing of antibodies<br />
development<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
8<br />
Remote outpatient clinics via telephone or video calls (tele-clinics)<br />
are increasingly utilized to reduce hospital congestion and seminal<br />
experiences in kidney transplantation have registered even higher<br />
attendance rates than conventional clinics in selected patients [10].<br />
Converting a proportion of outpatient clinics appointments to tele-clinics<br />
may reduce transplant population exposure to the virus. Numbers of<br />
visits (even tele-visits) can be reduced selecting only those patients<br />
with new symptoms or active issues, delaying well-being ones. A<br />
policy of remote management of immunosuppression by testing<br />
immunosuppressant level in local laboratories (then transmitted<br />
electronically) can be encouraged, thus relieving the workload on<br />
transplant centers.<br />
Virus tests and transplantation<br />
In transplant services, a delay or failure to diagnose SARS-CoV-2 infection<br />
in a donor may potentially produce disastrous consequences for the<br />
recipient and also increase the risk for health-workers [11]. In this context,<br />
the role of in vitro diagnostics is crucial to screen donors and recipients.<br />
An appropriate diagnostic strategy for the detection of virus infection<br />
involves collecting the correct specimen from the patient at the right time<br />
and performing an accurate and rapid laboratory test (Table 2).<br />
Reverse transcription-polymerase chain reaction<br />
The gold standard technique for detecting the SARS-CoV-2 infection<br />
is the real-time polymerase chain reaction (RT-PCR). This test has<br />
the advantage that the primers required can be produced as soon<br />
as the viral sequence is known. RT-PCR provides high levels of<br />
diagnostic sensitivity and specificity but the test protocol of nucleic<br />
acid amplification is complex and requires specialized instruments<br />
and technicians [12]. Although SARS-COV-2 RNA has been detected<br />
from a variety of respiratory sources, US Centers for Disease Control<br />
and Prevention recommends collecting only the upper respiratory NPS<br />
[13]. This indication is in accordance with Wang et al., that reported<br />
good detection rates of SARS-CoV-2 RNA in NPS (63% of the examined<br />
samples) [14]. SARS-CoV-2 RNA has been also detected from feces<br />
and blood specimens, although less reliably than from respiratory<br />
specimens. Higher viral loads have been detected soon after symptoms<br />
onset; thus, respiratory specimens should be collected within the first<br />
7 days. Missing the time-window of viral replication can cause false<br />
negative results [15, 16]. Several RT-PCR protocols for the detection of<br />
SARS-CoV-2 RNA have been released by the World Health Organization<br />
and nowadays are widely standardized. However, work overload and<br />
logistic difficulties to ship samples to the few specialized centers,<br />
lead to significant delays in response time (up to 4–5 days in remote<br />
hospitals) [17]. This has caused issues in transplant services where<br />
rapid tests are needed to accelerate clinical decision-making. Several<br />
new generation real-time RT-PCR protocols for the detection of SARS-<br />
COV-2 RNA have been recently developed. These assays are suitable<br />
for decentralized point-of-care use and allow obtaining reliable results<br />
within 1 h (actual state-of-the-art detection methods). One of these,<br />
Simplexa COVID-19 Direct (DiaSorin Molecular LLC, CA) received<br />
the FDA’s emergency use authorization and it is nowadays available in<br />
Italy. Simplexa incorporate nucleic acid extraction, amplification and<br />
detection together into an integrated system ensuring a simple, safe<br />
and highly qualitative test [18–20].<br />
Serology<br />
A recent study reported acute antibody responses to SARS-CoV-2 in<br />
285 patients and clarified that antibodies produced during the course<br />
of infection by symptomatic and asymptomatic patients can aid to the<br />
diagnosis of COVID-19 [21]. Immunoassays for detection of SARS-<br />
COV-2 immunoglobulin (Ig) M and IgG antibodies have proven to be<br />
highly specific and sensitive providing diagnostic evidence of infection<br />
in a few minutes. Moreover, the use of serology rapid tests could<br />
facilitate the diagnosis of SARS-CoV-2 infections when the molecular<br />
assays were performed unsatisfactorily [22, 23]. Several companies,<br />
driven by the growing demand of healthcare systems started to<br />
produce rapid immunoassays for SARS-CoV-2. The majority of these<br />
are solid phase immunochromatographic assays for the qualitative<br />
and differential detection in human whole blood, serum or plasma of<br />
IgG and IgM antibodies to SARS-CoV-2. Although the manufacturers<br />
guarantee an accuracy close to 100%, doubts exist in the scientific<br />
community about the time kinetics of humoral response and for the<br />
potential cross reactivity with other coronaviruses [24]. In our opinion,<br />
active surveillance with rapid serological tests may prove a good option<br />
for the screening of asymptomatic donors and recipients.
FEATURE<br />
Transplant benefit during the pandemic<br />
Limited resources allocation is the mainstay of patient care during<br />
catastrophes. When multiple casualties present at the same time,<br />
patients are triaged and treatments offered based on the chance<br />
of success. With the growing COVID-19 pandemic, the capacity of<br />
many intensive care units (ICU) has been saturated, which forced<br />
physicians to adopt a strict selection of patients who can be treated.<br />
Transplantation has always faced the issue of limited resources due<br />
to the scarcity of donors and the growing demand of organs. In liver<br />
transplantation, the concept of transplant benefit has gained wide<br />
acceptance in the last decade, in an effort to guarantee equity during<br />
organs allocation, counterbalancing the principles of utility (recipients<br />
with the highest chances of a good outcome) and urgency (recipients<br />
with the biggest need of transplantation) [25, 26].<br />
The widespread of COVID-19 has already caused a drastic reduction<br />
in organ donation and this is predicted to aggravate further in the<br />
next months. Times of further restraints stimulate reconsidering<br />
principles of allocation and adopt a pragmatic approach based on the<br />
available resources. A drop in the availability of blood products due<br />
to the reduction in blood donors has been registered too. Restricting<br />
transplants only to the sickest recipients (unbalancing towards the<br />
“urgency” principle) could address the need of patients at imminent<br />
risk of death from end-stage organ failure. However, it is not known<br />
how this will increase mortality rates on the waiting list for all other<br />
patients who are delayed (i.e., those at “the bottom of the list”). As an<br />
example, patients with model for end-stage liver disease (MELD) of<br />
30 have a 62% mortality rate without liver transplantation at 3 months<br />
while the rate drops to 25% with a MELD of 20. On the contrary,<br />
privileging liver transplant candidates with higher chances of success<br />
and therefore shorter hospital stay and lower consumption of blood<br />
transfusion (unbalancing towards the “utility” principle) would reduce<br />
the workload on ICUs, at the price of excluding the sickest candidates.<br />
Liver transplant recipients with MELD ≥30 have been shown to require<br />
about double the amount of perioperative blood transfusion and days<br />
of ICU stay compared to patients with MELD < 30 [27]. As happened in<br />
the past, it should be noted that wait-listed patients might be reluctant to<br />
undergo a transplant during the course of epidemics, especially those<br />
whose disease is not as severe to threaten life in the short-term [28].<br />
A “phased approach” to decreasing transplant activity has been<br />
proposed, with varying degrees of reduction depending on resource<br />
availability [29]. In addition, for the continuation of a transplant<br />
programme, a “clean path” within the ICU has to be maintained and not<br />
all hospitals might be in a condition to offer it.<br />
During the SARS outbreak in 2003 some transplant centers closed their<br />
activity temporarily and donor assessment guidelines were developed<br />
to mitigate the risk related to donor selection [30]. During the Ebola<br />
epidemic in 2014, the specifics of travel history of potential donors<br />
were thoroughly assessed by the organ procurement organizations.<br />
At that time, the high lethality of Ebola kept the number of affected<br />
people relatively low and the impact on organ donation was contained.<br />
The lack of effective treatments for Ebola stimulated the ethical debate<br />
around the value of the informed consent to transplantation in times of<br />
epidemics: a recipient might be willing to accept the risk of infection to<br />
gain the benefit of a new organ, however this does not contemplate the<br />
risk of infection spread to health-workers [31].<br />
In the United Kingdom, the national authority for transplantation has<br />
released clinical advice on donation acceptance criteria (deceased<br />
donors will be considered only if < 50 and < 60 years of age<br />
respectively for circulatory- and brain-dead donors). Most non-lifesaving<br />
transplant programmes such as pancreas and living-donor kidney<br />
have been put on hold [32]. In Switzerland, almost all non-lifesaving<br />
transplants have been suspended. Other countries have advised in<br />
favor of a case-by-case decision on both donation and transplantation,<br />
depending on local conditions.<br />
So far, most countries have reported a heterogeneous distribution of<br />
COVID-19 across their regions, with foci of high incidence of contagion<br />
causing major disruption to social life and healthcare. In a recently<br />
published article, Michaels et al. suggested to redistribute patients<br />
on the waiting list in endemic regions to less affected areas [33].<br />
Such approach offers the advantage of not penalizing patients on the<br />
waiting list only because of their geographical distribution, however,<br />
in a rapidly changing scenario, less affected areas may need to keep<br />
their resources available for possible sudden increases in hospital beds<br />
demand.<br />
Conclusions<br />
COVID-19 pandemic is an unprecedented life-changing crisis causing<br />
disruption in all the aspects of social life, especially for the wealthier<br />
economies of the world. As our health systems are built around patientcentered<br />
care, a cultural switch towards society over individual benefit<br />
seems mandatory in order not to run out of resources and guarantee<br />
the survival of our communities [34]. Stringent measures have been<br />
put in place to control the disease spread. Transplantation is one of the<br />
biggest advances in medical care and achievements in human history,<br />
a noble discipline that has crossed dangerous paths for the sake of its<br />
development. In this time of global crisis, the whole transplant community<br />
is called to join forces and develop strategies to mitigate risks and<br />
continue delivering the best possible results with the available resources<br />
to the multitude of patients awaiting organs from all over the world.<br />
Abbreviations<br />
COVID-19: Coronavirus disease-19; ICU: Intensive care unit; Ig: Immunoglobulin;<br />
MELD: Model for end-stage liver disease; MERS: Middle East respiratory<br />
syndrome; NPS: Naso-pharyngeal swab; RNA: Ribonucleic acid; RT-PCR: Reverse<br />
transcription-polymerase chain reaction; SARSCoV2: Severe acute respiratory<br />
syndrome Coronavirus 2<br />
Acknowledgements<br />
None.<br />
Authors’ contributions<br />
GS, GB, DG and QL were responsible for the conception, design and analysis<br />
of the study; GS, GB and DG were involved with the writing of the manuscript,<br />
collection and interpretation of data; QL was involved in the writing, reviewing and<br />
editing of the manuscript. All authors have read and approved the manuscript.<br />
Funding<br />
This study was not supported by any funding.<br />
Availability of data and materials<br />
The data used and analyzed during the current study are extrapolated and<br />
available from the cited articles as listed in the “Reference” section. If requested by<br />
the editors, we will provide the data and information on which the conclusions of<br />
this manuscript are based.<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
9
FEATURE<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
Ethics approval and consent to participate<br />
The study is a narrative review and represents the Authors’ opinions on the<br />
subject. Direct patient data collection and participants consent were not<br />
necessary.<br />
Consent for publication<br />
The study is a narrative review and represents the Authors’ opinions on the<br />
subject. Direct patient data collection and consent to publish were not necessary.<br />
Competing interests<br />
GS and QL are members of the editorial board (Associate Editor) of BMC<br />
<strong>Gastroenterology</strong>. GB and DG have no conflicts of interest to declare about the<br />
present study.<br />
Author details<br />
1<br />
General Surgery and Liver Transplantation, Fondazione Policlinico Universitario A.<br />
Gemelli IRCCS, Largo Agostino Gemelli 8, 00168 Rome, Italy.<br />
2<br />
Centre for the Study and Treatment of Psoriasis, Department of Clinical<br />
Dermatology, San Gallicano Dermatological Institute, IRCCS, Rome, Italy.<br />
3<br />
Hepatobiliary and Organ Transplantation Unit, Sapienza University of Rome,<br />
Umberto I Polyclinic of Rome, Rome, Italy.<br />
Received: 8 May <strong>2020</strong> Accepted: 27 July <strong>2020</strong><br />
References<br />
1. Coronavirus disease 2019 (COVID-19) Situation Report – 104. https://www.<br />
who.int/docs/default-source/coronaviruse/situation-reports/<strong>2020</strong>0503-covid-<br />
19-sitrep-104.pdf?sfvrsn=53328f46_2 Accessed 3 May <strong>2020</strong>.<br />
2. C_17_cntAvvisi_229_0_file.pdf. http://www.trapianti.salute.gov.it/imgs/C_17_<br />
cntAvvisi_229_0_file.pdf. Accessed 4 Apr <strong>2020</strong>.<br />
3. C_17_cntAvvisi_234_0_file.pdf. http://www.trapianti.salute.gov.it/imgs/C_17_<br />
cntAvvisi_234_0_file.pdf. Accessed 4 Apr <strong>2020</strong>.<br />
4. Team TNCPERE. The epidemiological characteristics of an outbreak of 2019<br />
novel coronavirus diseases (COVID-19) — China, <strong>2020</strong>. China CDC Wkly.<br />
<strong>2020</strong>;2(8):113–22.<br />
5. EpiCentro. Coronavirus | Istituto Superiore di Sanità. https://www.epicentro.<br />
iss.it/coronavirus/. Accessed 3 May <strong>2020</strong>.<br />
6. Polak WG, Fondevila C, Karam V, et al. Impact of COVID-19 on liver<br />
transplantation in Europe: alert from an early survey of European liver and<br />
intestine transplantation association (ELITA) and European liver transplant<br />
registry (ELTR). Transpl Int. <strong>2020</strong>. doi:https://doi.org/10.1111/tri.13680.<br />
Published online July 1, <strong>2020</strong>.<br />
7. Akdur A, Karakaya E, Ayvazoglu Soy EH, et al. Coronavirus disease<br />
(COVID-19) in kidney and liver transplant patients: a single-center experience.<br />
Exp Clin Transplant. <strong>2020</strong>;18(3):270–4. https://doi.org/10.6002/ect.<strong>2020</strong>.0193.<br />
8. Lai Q, Melandro F, Rossi M, Ruberto F, Pugliese F, Mennini G. Role of<br />
perfusion machines in the setting of clinical liver transplantation: a qualitative<br />
systematic review. Clin Transpl. 2018;32(8):e13310. https://doi.org/10.1111/<br />
ctr.13310.<br />
9. Yeung JC, Krueger T, Yasufuku K, et al. Outcomes after transplantation of<br />
lungs preserved for more than 12 h: a retrospective study. Lancet Respir Med.<br />
2017;5(2):119–24. https://doi.org/10.1016/S2213-2600(16)30323-X.<br />
10. Udayaraj UP, Watson O, Ben-Shlomo Y, et al. Establishing a tele-clinic<br />
service for kidney transplant recipients through a patient-codesigned quality<br />
improvement project. BMJ Open Qual. 2019;8(2). https://doi.org/10.1136/<br />
bmjoq-2018-000427.<br />
11. Lai Q, Spoletini G, Bianco G, et al. SARS-CoV2 and immunosuppression:<br />
A double-edged sword. Transpl Infect Dis. <strong>2020</strong>:e13404. https://doi.<br />
org/10.1111/tid.13404 Published online July 8, <strong>2020</strong>.<br />
12. Loeffelholz MJ, Tang Y-W. Laboratory diagnosis of emerging human<br />
coronavirus infections - the state of the art. Emerg Microbes Infect. <strong>2020</strong>:1–<br />
26. https://doi.org/10.1080/22221751.<strong>2020</strong>.1745095 Published online March<br />
20, <strong>2020</strong>.<br />
13. CDC. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control<br />
and Prevention. Published February 11, <strong>2020</strong>. https://www.cdc.gov/<br />
coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html Accessed 27<br />
Mar <strong>2020</strong>.<br />
14. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of<br />
clinical specimens. JAMA <strong>2020</strong>. doi:https://doi.org/10.1001/jama.<strong>2020</strong>.3786.<br />
Published online March 11, <strong>2020</strong>.<br />
15. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory<br />
specimens of infected patients. N Engl J Med. <strong>2020</strong>;382(12):1177–9. https://<br />
doi.org/10.1056/NEJMc2001737.<br />
16. Wu JT, Leung K, Bushman M, et al. Estimating clinical severity of COVID-19<br />
from the transmission dynamics in Wuhan, China Nat Med <strong>2020</strong>:1–5.<br />
doi:https://doi.org/10.1038/s41591-020-0822-7. Published online March 19,<br />
<strong>2020</strong>.<br />
17. National laboratories. https://www.who.int/emergencies/diseases/<br />
novelcoronavirus-2019/technical-guidance/laboratory-guidance. Accessed<br />
March 27, <strong>2020</strong>.<br />
18. Chan JF-W, Yip CC-Y, To KK-W, et al. Improved molecular diagnosis of<br />
COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel<br />
real-time reverse transcription-polymerase chain reaction assay validated<br />
in vitro and with clinical specimens. J Clin Microbiol <strong>2020</strong>. doi:https://doi.<br />
org/10.1128/JCM.00310-20. Published online March 4, <strong>2020</strong>.<br />
19. Lieberman JA, Pepper G, Naccache SN, Huang M-L, Jerome KR, Greninger<br />
AL. Comparison of commercially available and laboratory developed assays<br />
for in vitro detection of SARS-CoV-2 in clinical laboratories. J Clin Microbiol.<br />
<strong>2020</strong>. https://doi.org/10.1128/JCM.00821-20 Published online April 29, <strong>2020</strong>.<br />
20. Rhoads DD, Cherian SS, Roman K, Stempak LM, Schmotzer CL, Sadri<br />
N. Comparison of Abbott ID now, Diasorin Simplexa, and CDC FDA EUA<br />
methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal<br />
swabs from individuals diagnosed with COVID-19. J Clin Microbiol. <strong>2020</strong>.<br />
doi:https://doi.org/10.1128/JCM.00760-20. Published online April 17, <strong>2020</strong>.<br />
21. Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in<br />
patients with COVID-19. Nat Med <strong>2020</strong>. doi:https://doi.org/10.1038/s41591-<br />
020-0897-1. Published online April 29, <strong>2020</strong>.<br />
22. Guo L, Ren L, Yang S, et al. Profiling early Humoral response to diagnose<br />
novel coronavirus disease (COVID-19). Clin Infect Dis <strong>2020</strong>. doi:https://doi.<br />
org/10.1093/cid/ciaa310. Published online March 21.<br />
23. Sheridan C. Fast, portable tests come online to curb coronavirus pandemic.<br />
Nat Biotechnol, <strong>2020</strong>. doi:https://doi.org/10.1038/d41587-020-00010-2.<br />
Published online March 23, <strong>2020</strong>.<br />
24. Zhang W, Du R-H, Li B, et al. Molecular and serological investigation of<br />
2019-nCoV infected patients: implication of multiple shedding routes.<br />
Emerg Microbes Infect. <strong>2020</strong>;9(1):386–9. https://doi.org/10.1080/22221751.<br />
<strong>2020</strong>.1729071.<br />
25. Schaubel DE, Guidinger MK, Biggins SW, et al. Survival benefit-based<br />
deceased-donor liver allocation. Am J Transplant. 2009;9(4 Pt 2):970–81.<br />
https://doi.org/10.1111/j.1600-6143.2009.02571.x.<br />
26. Vitale A, Volk ML, De Feo TM, et al. A method for establishing allocation equity<br />
among patients with and without hepatocellular carcinoma on a common liver<br />
transplant waiting list. J Hepatol. 2014;60(2):290–7. https://doi.org/10.1016/j.<br />
jhep.2013.10.010.<br />
27. Schlegel A, Linecker M, Kron P, et al. Risk assessment in high- and low-MELD<br />
liver transplantation. Am J Transplant. 2017;17(4):1050–63. https://doi.<br />
org/10.1111/ajt.14065.<br />
28. Chui AKK, Rao ARN, Chan HLY, Hui AY. Impact of severe acute respiratory<br />
syndrome on liver transplantation service. Transplant Proc. 2004;36(8):2302–<br />
3. https://doi.org/10.1016/j.transproceed.2004.08.018.<br />
29. Kumar D, Manuel O, Natori Y, et al. COVID-19: a global transplant perspective<br />
on successfully navigating a pandemic. Am J Transplant. <strong>2020</strong>. doi:https://<br />
doi.org/10.1111/ajt.15876. Published online March 23, <strong>2020</strong>.<br />
30. Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory<br />
Syndrome (SARS) in a liver transplant recipient and guidelines for<br />
donor SARS screening. Am J Transplant. 2003;3(8):977–81. https://doi.<br />
org/10.1034/j. 1600-6143.2003.00197.x.<br />
31. Kaul DR, Mehta AK, Wolfe CR, Blumberg E, Green M. Ebola virus disease:<br />
implications for solid organ transplantation. Am J Transplant. 2015;15(1):5–6.<br />
https://doi.org/10.1111/ajt.13093.<br />
32. COVID-19: Advice for Clinicians - ODT Clinical - NHS Blood and Transplant.<br />
https://www.odt.nhs.uk/deceased-donation/covid-19-advice-for-clinicians/.<br />
Accessed 26 Mar <strong>2020</strong>.<br />
33. Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease<br />
2019: Implications of emerging infections for transplantation. Am J Transplant<br />
<strong>2020</strong>. doi:https://doi.org/10.1111/ajt.15832. Published online February 24,<br />
<strong>2020</strong>.<br />
34. Nacoti M, Ciocca A, Giupponi A, et al. At the Epicenter of the Covid-19<br />
Pandemic and Humanitarian Crises in Italy: Changing Perspectives on<br />
Preparation and Mitigation. NEJM Catal. 1(2). https://doi.org/10.1056/<br />
CAT.20.0080.<br />
Publisher’s Note<br />
Springer Nature remains neutral with regard to jurisdictional claims in published<br />
maps and institutional affiliations.<br />
10
FEATURE<br />
How can you reduce the risk to<br />
your Crohn’s disease patients<br />
of serious COVID-19 disease? 1<br />
Prescribe<br />
Entocort ® CR:<br />
classified by the<br />
BSG as lowest risk<br />
of serious COVID-19<br />
disease, compared<br />
to higher-risk<br />
prednisolone 1<br />
Entocort ® CR: BSG-recommended control patients can count on 1–3<br />
Entocort ® CR is indicated for the induction<br />
of remission in adults with mild to<br />
moderate active Crohn’s disease affecting<br />
the ileum and/or the ascending colon. 4<br />
ENTOCORT CR 3mg Capsules (budesonide) -<br />
Prescribing Information<br />
Please consult the Summary of Product Characteristics<br />
(SmPC) for full prescribing Information<br />
Presentation: Hard gelatin capsules for oral administration<br />
with an opaque, light grey body and an opaque, pink cap<br />
marked CIR 3mg in black radial print. Contains 3mg<br />
budesonide. Indications: Induction of remission in patients<br />
with mild to moderate Crohn’s disease affecting the ileum<br />
and/or the ascending colon. Induction of remission in patients<br />
with active microscopic colitis. Maintenance of remission in<br />
patients with microscopic colitis. Dosage and<br />
administration: Active Crohn’s disease (Adults): 9mg once<br />
daily in the morning for up to eight weeks. Full effect achieved<br />
in 2-4 weeks. When treatment is to be discontinued, dose<br />
should normally be reduced in final 2-4 weeks. Active<br />
microscopic colitis (Adults): 9mg once daily in the morning.<br />
Maintenance of microscopic colitis (Adults): 6mg once daily in<br />
the morning, or the lowest effective dose. Paediatric<br />
population: Not recommended. Older people: No special<br />
dose adjustment recommended. Swallow whole with water.<br />
Do not chew. Contraindications: Hypersensitivity to the<br />
active substance or any of the excipients. Warnings and<br />
Precautions: Side effects typical of corticosteroids may<br />
occur. Visual disturbances may occur. If a patient presents<br />
with symptoms such as blurred vision or other visual<br />
disturbances they should be considered for referral to an<br />
ophthalmologist for evaluation of the possible causes.<br />
Systemic effects may include glaucoma and when prescribed<br />
at high doses for prolonged periods, Cushing’s syndrome,<br />
adrenal suppression, growth retardation, decreased bone<br />
mineral density and cataract. Caution in patients with infection,<br />
hypertension, diabetes mellitus, osteoporosis, peptic ulcer,<br />
glaucoma or cataracts or with a family history of diabetes or<br />
glaucoma. Particular care in patients with existing or previous<br />
history of severe affective disorders in them or their first<br />
degree relatives. Caution when transferring from<br />
glucocorticoid of high systemic effect to Entocort CR. Chicken<br />
pox and measles may have a more serious course in patients<br />
on oral steroids. They may also suppress the HPA axis and<br />
reduce the stress response. Reduced liver function may<br />
increase systemic exposure. When treatment is discontinued,<br />
reduce dose over last 2-4 weeks. Concomitant use of CYP3A<br />
inhibitors, such as ketoconazole and cobicistat-containing<br />
products, is expected to increase the risk of systemic side<br />
effects and should be avoided unless the benefits outweigh<br />
the risks. Excessive grapefruit juice may increase systemic<br />
exposure and should be avoided. Patients with fructose<br />
intolerance, glucose-galactose malabsorption or sucroseisomaltase<br />
insufficiency should not take Entocort CR. Monitor<br />
height of children who use prolonged glucocorticoid therapy<br />
for risk of growth suppression. Interactions: Concomitant<br />
colestyramine may reduce Entocort CR uptake. Concomitant<br />
oestrogen and contraceptive steroids may increase effects.<br />
CYP3A4 inhibitors may increase systemic exposure. CYP3A4<br />
inducers may reduce systemic exposure. May cause low<br />
values in ACTH stimulation test. Fertility, pregnancy and<br />
lactation: Only to be used during pregnancy when the<br />
potential benefits to the mother outweigh the risks for the<br />
foetus. May be used during breast feeding. Adverse<br />
reactions: Common: Cushingoid features, hypokalaemia,<br />
behavioural changes such as nervousness, insomnia, mood<br />
swings and depression, palpitations, dyspepsia, skin reactions<br />
(urticaria, exanthema), muscle cramps, menstrual disorders.<br />
Uncommon: anxiety, tremor, psychomotor hyperactivity.<br />
Rare: aggression, glaucoma, cataract, blurred vision,<br />
ecchymosis. Very rare: Anaphylactic reaction, growth<br />
retardation. Prescribers should consult the summary of<br />
product characteristics in relation to other adverse reactions.<br />
Marketing Authorisation Numbers, Package<br />
Quantities and basic NHS price: PL 36633/0006. Packs of<br />
50 capsules: £37.53. Packs of 100 capsules: £75.05. Legal<br />
category: POM. Marketing Authorisation Holder: Tillotts<br />
Pharma UK Ltd, The Stables, Wellingore Hall, Wellingore,<br />
Lincoln, LN5 0HX. Date of preparation of PI: February <strong>2020</strong><br />
Adverse events should be reported.<br />
Reporting forms and information can be found at<br />
https://yellowcard.mhra.gov.uk. Adverse events<br />
should also be reported to Tillotts Pharma UK Ltd.<br />
Tel: 01522 813500.<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
References: 1. Kennedy NA et al. Gut <strong>2020</strong>; 0: 1–7. 2. Campieri M<br />
et al. Gut 1997; 41(2): 209–214. 3. Lamb CA et al. Gut 2019; 0: 1–106.<br />
4. Entocort ® CR 3 mg capsules – Summary of Product Characteristics.<br />
Date of preparation: July <strong>2020</strong>. PU-00377.<br />
11
FEATURE<br />
RETHINKING HOW WE TREAT<br />
CONSTIPATION IN THE UK<br />
Professor Anton Emmanuel, Consultant Gastroenterologist at UCLH and the National Hospital for Neurology & Neurosurgery<br />
In 2018 alone, the UK’s national health system (NHS) saw more<br />
than 52,000 emergency hospital admissions for constipation –<br />
the cost of which adds up to a staggering £71 million per year. 1<br />
Managing such avoidable costs out of the healthcare system<br />
is now even more of a priority given the effects of the Covid-19<br />
pandemic and its aftermath. The NHS urgently needs nonconsultation<br />
pathways to transform sufferers’ lives, yet also<br />
minimise impact on precious resources.<br />
Pyramid” which clearly plots an effective course of treatment. The pyramid<br />
diagram shows that effective treatment of constipation should be done in<br />
incremental stages whereby patients are moved further up the treatment<br />
ladder until their condition comes under control. Starting from the base<br />
layer of the pyramid, patients will first begin treatment with the most<br />
conservative options such as adjustment of diet and fluid intake, lifestyle<br />
alteration oral medications including stool softeners and laxatives, digital<br />
stimulation, suppositories and biofeedback.<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
We have to start from the question, “why is an easily manageable and<br />
treatable condition like constipation resulting in such a high number<br />
of unplanned emergency hospital admissions?”. The emergency<br />
admission numbers demonstrate the urgent need to revise the current<br />
treatment for constipation and prevent so many cases from escalating<br />
into unwanted admissions. Unplanned hospital visits could certainly be<br />
reduced if symptoms were identified and dealt with at the primary care<br />
stage, but many healthcare professionals lack the right information to<br />
effectively treat the condition. In fact, beyond standard interventions<br />
such as laxatives and dietary changes, there is little supporting<br />
material to guide healthcare professionals through the next stages of<br />
treatment. Drawing on the Bowel Interest Group’s (BIG) newly published<br />
management pathway, this article seeks to help bridge this information<br />
gap by underlining best practices in bowel management.<br />
One of the first barriers to effective treatment of constipation is its<br />
perceived taboo nature. The stigma attached to constipation means that<br />
people are suffering in silence needlessly until the condition becomes<br />
too difficult to bear. Patients are reluctant to share symptoms with their<br />
doctor at the early stages of the condition – with as many as one in<br />
five stating they would be too embarrassed to talk about it at all 2 – and<br />
therefore receive less timely treatment than they should. In parallel, the<br />
high prevalence of these symptoms results in it being perceived by some<br />
healthcare professionals as low risk and of minor significance, further<br />
delaying treatment. Unfortunately, this potentially sets the stage for more<br />
invasive interventions and unwanted hospital admissions in the long run.<br />
With bowel behaviour serving as an important indicator of our health,<br />
it is crucial that we break down this wall and promote more open<br />
conversations about bowel health. Greater awareness about constipation,<br />
as well as correct advice and treatment, will help to make people feel<br />
more comfortable talking to their GP. Without intervention, people with<br />
bowel disorders can suffer from reduced quality of life including feelings<br />
of embarrassment, anxiety and depression – as well as a number of<br />
unwanted side-effects such as urinary tract infections (UTIs). Failure to<br />
deal with symptoms promptly can lead to more complex problems such<br />
as haemorrhoids, anal fissures or rectal prolapse, so it is important to<br />
diagnose and treat the condition as early as possible.<br />
The next step is ensuring that healthcare professionals themselves are<br />
sufficiently informed about treatment options for constipation. In support,<br />
the Bowel Interest Group has developed a ‘Bowel Dysfunction Treatment<br />
If these standard interventions are not effective within the prescribed<br />
three-month period, patients would typically then progress onto the next<br />
stage of treatment: minimally invasive treatment options such as transanal<br />
irrigation (TAI). It is important that the prescribed length of treatment is<br />
consistently adhered to at every level and that patients are moved up the<br />
pyramid once the given timeframe has elapsed. This ensures that patients<br />
suffering from constipation can reach the appropriate therapy level and<br />
resume their normal lives as quickly as possible. The upper layers of the<br />
treatment pyramid are comprised of more invasive treatment options such<br />
as nerve stimulation implants and surgical colonic irrigation. Finally, the<br />
last recourse if these are ineffective, is the creation of a permanent stoma<br />
– which constitutes the peak of the pyramid.<br />
Another important consideration for GPs and Clinical Commissioning<br />
Groups is the cost associated with each therapy. BIG’s pyramid diagram<br />
provides this valuable information, sub-categorized into the one-off cost,<br />
the annual cost and the 7-year cost for each treatment. For instance,<br />
while the cost of standard starting treatment should amount to £2,539,<br />
this figure can reach up to £32,298 over seven years if practitioners do not<br />
progress their patients up the pyramid towards more effective treatment<br />
within the recommended timeframe. 3 Respecting the designated<br />
timeframe for each treatment echelon therefore makes sense from both a<br />
patient-outcome perspective as well as from a financial outlook.<br />
Bowel management in the UK is in need of urgent reform, despite<br />
pockets of excellence scattered across the country. <strong>Today</strong>, constipation<br />
is often perceived as low priority despite having a hugely detrimental<br />
impact on the patients it affects, as well as the financial burden that its<br />
ineffective treatment imposes on the healthcare system. Having the right<br />
pathways in place at the primary level is crucial to ensuring patients<br />
do not require unplanned emergency interventions within already<br />
overstretched facilities. The Covid-19 experience has served to highlight<br />
even further the requirement for non-consultation pathways to transform<br />
sufferers’ lives, yet also minimise impact on precious resources.<br />
Securing these outcomes will take a nationwide effort to rebuild our<br />
understanding of the management of constipation.<br />
Please find the Bowel Interest Group’s full report - Dealing with Chronic<br />
Constipation: Information for General Practitioners:<br />
https://bowelinterestgroup.co.uk/resources/dealing-with-chronicconstipation-information-for-general-practitioners/<br />
12<br />
1<br />
Bowel Interest Group, Cost of Constipation Report, Second edition, 2019<br />
2<br />
Ibid<br />
3<br />
Bowel Interest Group, Dealing with Chronic Constipation: Information for General Practitioners, <strong>2020</strong>
The only licensed treatment for the<br />
ADVERTORIAL FEATURE<br />
reduction in recurrence of overt<br />
hepatic encephalopathy (OHE) 1<br />
At home they<br />
are still at risk;<br />
…TARGAXAN ®<br />
rifaximin-α<br />
reduces the risk<br />
of recurrence<br />
of overt hepatic<br />
encephalopathy. 2<br />
Long-term secondary prophylaxis in hepatic<br />
encephalopathy (HE) 3<br />
UK&IE Prescribing Information: Targaxan 550mg (rifaximin-α)<br />
REFER TO FULL SUMMARY OF PRODUCT CHARACTERISTICS (SmPC)<br />
BEFORE PRESCRIBING<br />
Presentation: Film-coated tablet containing rifaximin 550 mg.<br />
Uses: Targaxan is indicated for the reduction in recurrence of episodes<br />
of overt hepatic encephalopathy in patients ≥ 18 years of age.<br />
Dosage and administration: Adults 18 years of age and over: 550 mg<br />
twice daily, with a glass of water, with or without food for up to<br />
6 months. Treatment beyond 6 months should be based on risk benefit<br />
balance including those associated with the progression of the patients<br />
hepatic dysfunction. No dosage changes are necessary in the elderly or<br />
those with hepatic insufficiency. Use with caution in patients with renal<br />
impairment.<br />
Contraindications: Contraindicated in hypersensitivity to rifaximin,<br />
rifamycin-derivatives or to any of the excipients and in cases of intestinal<br />
obstruction.<br />
Warnings and precautions for use: The potential association of<br />
rifaximin treatment with Clostridium difficile associated diarrhoea and<br />
pseudomembranous colitis cannot be ruled out. The administration<br />
of rifaximin with other rifamycins is not recommended. Rifaximin<br />
may cause a reddish discolouration of the urine. Use with caution<br />
in patients with severe (Child-Pugh C) hepatic impairment and in<br />
patients with MELD (Model for End-Stage Liver Disease) score > 25.<br />
In hepatic impaired patients, rifaximin may decrease the exposure<br />
of concomitantly administered CYP3A4 substrates (e.g. warfarin,<br />
antiepileptics, antiarrhythmics, oral contraceptives). Both decreases and<br />
increases in international normalized ratio (in some cases with bleeding<br />
events) have been reported in patients maintained on warfarin and<br />
prescribed rifaximin. If co-administration is necessary, the international<br />
normalized ratio should be carefully monitored with the addition or<br />
withdrawal of treatment with rifaximin. Adjustments in the dose of<br />
oral anticoagulants may be necessary to maintain the desired level of<br />
anticoagulation. Ciclosporin may increase the rifaximin C max<br />
Pregnancy and lactation: Rifaximin is not recommended during<br />
pregnancy. The benefits of rifaximin treatment should be assessed<br />
against the need to continue breastfeeding.<br />
Side effects: Common effects reported in clinical trials are dizziness,<br />
headache, depression, dyspnoea, upper abdominal pain, abdominal<br />
distension, diarrhoea, nausea, vomiting, ascites, rashes, pruritus,<br />
muscle spasms, arthralgia and peripheral oedema. Other effects that<br />
have been reported include: Clostridial infections, urinary tract<br />
infections, candidiasis, pneumonia cellulitis, upper respiratory tract<br />
infection and rhinitis. Blood disorders (e.g. anaemia,<br />
thrombocytopenia). Anaphylactic reactions, angioedemas,<br />
hypersensitivity. Anorexia, hyperkalaemia and dehydration. Confusion,<br />
sleep disorders, balance disorders, convulsions, hypoesthesia,<br />
memory impairment and attention disorders. Hypotension,<br />
hypertension and fainting. Hot flushes. Breathing difficulty, pleural<br />
effusion, COPD. Gastrointestinal disorders and skin reactions. Liver<br />
function test abnormalities. Dysuria, pollakiuria and proteinuria.<br />
Oedema. Pyrexia. INR abnormalities. Prescribers should consult the<br />
SmPC in relation to all adverse reactions.<br />
UNITED KINGDOM<br />
Legal category: POM<br />
Cost: Basic NHS price £259.23 for 56 tablets<br />
Marketing Authorisation holder: Norgine Pharmaceuticals Limited,<br />
Norgine House, Widewater Place, Moorhall Road, Harefield, Uxbridge,<br />
UB9 6NS, UK<br />
Marketing Authorisation number: PL 20011/0020<br />
IRELAND<br />
Legal category: Prescription only<br />
Cost: €262.41 for 56 tablets<br />
Marketing Authorisation holder: Norgine B.V. Antonio Vivaldistraat 150,<br />
1083 HP, Amsterdam, Netherlands<br />
Marketing Authorisation number: PA 1336/009/001<br />
For further information contact: Norgine Pharmaceuticals Limited,<br />
Norgine House, Moorhall Road, Harefield, Middlesex UB9 6NS<br />
Telephone: 01895 826 606 E-mail: Medinfo@norgine.com<br />
Ref: UK/XIF5/0519/0509<br />
Date of preparation: May 2019<br />
United Kingdom<br />
Adverse events should be reported. Reporting forms and<br />
information can be found at www.mhra.gov.uk/yellowcard.<br />
Adverse events should also be reported to Medical<br />
Information at Norgine Pharmaceuticals Ltd on:<br />
Tel. +44 (0)1895 826 606 Email Medinfo@norgine.com<br />
Ireland<br />
Healthcare professionals are asked to report any suspected<br />
adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace,<br />
IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517.<br />
Website: www.hpra.ie; E-mail: medsafety@hpra.ie.<br />
Adverse events should also be reported to Medical Information<br />
at Norgine Pharmaceuticals Ltd on: Tel. +44 (0)1895 826 606<br />
Email Medinfo@norgine.com<br />
References:<br />
1. National Institute for Health and Care Excellence. Rifaximin for<br />
preventing episodes of overt hepatic encephalopathy: appraisal<br />
guidance TA337 for rifaximin. Available from: http://www.nice.org.<br />
uk/guidance/ta337<br />
2. TARGAXAN ® 550 Summary of Product Characteristics. Available<br />
for the UK from: https://www.medicines.org.uk/emc Available for<br />
Ireland from: www.medicines.ie<br />
3. Mullen KD, et al. Clin Gastroenterol Hepatol 2014;12(8):1390-97.<br />
Product under licence from Alfasigma S.p.A. TARGAXAN is a<br />
registered trademark of the Alfasigma group of companies, licensed<br />
to the Norgine group of companies. NORGINE and the sail logo are<br />
registered trademarks of the Norgine group of companies.<br />
UK/XIF5/0919/0549<br />
Date of preparation: October 2019.<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
13
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
NHS trusts with:<br />
2WW Urgent referrals<br />
Routine referrals<br />
ADVERTORIAL FEATURE<br />
Surveillance cases<br />
Bowel cancer screening services<br />
ENDOSCOPY ALTERNATIVES IN A TIME OF COVID:<br />
NHS Facility NHS Staff NHS<br />
INNOVATIVE THINKING AND DIFFERENT WAYS OF WORKING<br />
processes<br />
TO CLEAR NHS TRUSTS WAITING LISTS<br />
Enhanced sedation (Propofol) lists<br />
Additionally, we can support Direct Access<br />
and Rapid Access endoscopy referrals by<br />
working with the local clinical leads to agree<br />
strong governance for the management of<br />
these patients.<br />
This quarter we explore the potential for Transnasal endoscopy as a<br />
new technological alternative which may assist Trusts in managing<br />
the significant spike in diagnostics demand arising from COVID 19.<br />
Transnasal endoscopy, or TNE, is an upper GI endoscopy method which<br />
is performed by the nasal route (rather than the oral route) using a thin<br />
Criteria & Quality<br />
endoscope less than 6 mm in diameter. This technique has been shown<br />
to improve patient tolerance and is more convenient.<br />
We select Endoscopists with an endoscopy<br />
orientated career path and performance<br />
measures above the national average. JAG<br />
audit data is constantly monitored to ensure<br />
has prevented successful internal review.<br />
ongoing quality. Furthermore, we have a<br />
Sedation is not required during Transnasal endoscopy and therefore<br />
clinical governance department that is crucial<br />
to maintaining quality and safety but also<br />
provides support to both Endoscopists and<br />
ensuring diagnostics continue while, importantly, being separated<br />
the from units COVID within red zones. which Recovery we times work. are short and the patient is<br />
Unsedated conventional oral gastroscopy (c-OGD) commonly causes<br />
gagging, retching and nausea which are avoided with TNE due to limited<br />
stimulation of the tongue and soft palate, thus saving treatment time and<br />
occasional repeat patient visits where physical rejection of the camera<br />
nursing staff will not be required for monitoring patient vital signs, offering<br />
an immediate cost-saving. The procedure can furthermore be carried<br />
out in an outpatient setting with a smaller estates footprint, importantly<br />
able to leave the room and hospital immediately once the procedure<br />
is completed and without the need for further monitoring or recovery<br />
We provide tailored solutions to manage<br />
capacity from straight forward supply of staff<br />
to a team based managed solution to a full<br />
patient pathway including pathology review.<br />
facilities (Gorelick et al. 2001). There are a number of cost analyses<br />
clearly demonstrating the cost savings for TNE (Wellenstein et al. 2019;<br />
Anon n.d.; Atar and Kadayifci 2014), and these come from a combination<br />
of decreasing the cost and total duration of endoscopic procedures,<br />
increased capacity and reduced staff requirements and all while allowing<br />
deployment in safe, manageable outpatient settings.<br />
Accuracy of Diagnosis<br />
Our commitment to improving the<br />
NHS experience<br />
conventional endoscopes, thus maintaining the diagnostic accuracy.<br />
Like Current the data NHS suggests Trusts that TNE we has work better with, patient tolerance patient when<br />
care is at the centre of everything we do. By<br />
using any spare weekend capacity within a<br />
Trust, the 18 Week Support insourcing teams<br />
Training & Deployment<br />
are able to see a high volume of patients<br />
in a short space of time, in the familiar<br />
surrounding of the NHS Trust.<br />
To date, data suggests that there is preservation of the image quality of<br />
compared to unsedated endoscopy (Garcia et al. 2003; Parker et al. 2016;<br />
Schuldt et al. 2019). Nasal pain is the most significant symptom associated<br />
with endoscopic procedures but can be reduced with nasal pre-treatment.<br />
Transnasal endoscopes are very similar to standard or slim endoscopes<br />
except for their outer diameter, which is usually less than 6 mm, and a<br />
smaller working channel, of only 2 mm in diameter. The disadvantage<br />
of this smaller calibre working channel is that only specialist paediatric<br />
biopsy forceps can be used to take tissue samples. There is the risk that<br />
An ethical company<br />
We’re an ethical and transparent company<br />
that’s financially accountable and financially<br />
responsible.<br />
rooms is not required.<br />
We’re committed to the NHS<br />
14and the delivery of high-quality care, and to<br />
helping Trusts reduce RTT waiting times.<br />
histological analysis may be impaired with smaller tissue samples.<br />
Implementation of TNE requires procurement of the endoscopes and if<br />
necessary a dedicated processor. The fact that these procedures can<br />
be carried out in outpatient settings means that refurbishing hospital<br />
Clinical team<br />
There is no formal training program for TNE, but all endoscopists<br />
undertaking Trans-nasal endoscopy procedures must have JAG<br />
certification for diagnostic UGI endoscopy (oral route). However, there<br />
is a requirement to understand the nasal anatomy and how to deal with<br />
complications. There are also subtle differences to the techniques required<br />
to negotiate some aspects of the anatomy, particularly large hiatus hernias<br />
Happy patient<br />
and passage through to D2. There are training courses available, which<br />
JAG strongly recommends clinicians attend. It is recommended that<br />
ENT surgeons should be involved at local service level to understand<br />
the anatomical approach and managing complications, and to provide<br />
mentoring. It is advised that a minimum of 20 full procedures are observed<br />
Who we’re looking for<br />
and competencies met before independent practice.<br />
We are interested in meeting with Consultant<br />
Summary<br />
Gastroenterologists, senior nurses and clinical<br />
nurse specialists throughout the UK.<br />
Transnasal endoscopy offers Trusts considerable advantages and<br />
flexibility during this time of COVID. TNE can be safely and easily<br />
deployed, including in outpatient settings which makes it easy to keep<br />
Our remuneration package is second to<br />
none and is per session rather than per case<br />
which allows our teams to work in a safe and<br />
Bibliography<br />
calm environment’<br />
diagnostic patients separate from COVID red zones; accurate diagnostic<br />
results can be delivered with smaller teams and with reduced impact on<br />
the patient; and special TNE training and deployment needs are limited.<br />
Anon Cost Savings of Transnasal Endoscopy Versus Standard Endosco...: Official<br />
journal of the American College of <strong>Gastroenterology</strong> | ACG [Online]. Available at:<br />
https://journals.lww.com/ajg/Fulltext/2008/09001/Cost_Savings_of_Transnasal_<br />
Endoscopy_Versus.1037.aspx About you [Accessed: 24 August <strong>2020</strong>a].<br />
Atar, M. and Kadayifci, A. 2014. Transnasal endoscopy: Technical considerations,<br />
advantages and limitations. World journal of gastrointestinal endoscopy 6(2), pp. 41–48.<br />
If you have an excellent NHS record and<br />
Garcia, R.T., Cello, J.P., Nguyen, M.H., et al. 2003. Unsedated ultrathin EGD is well<br />
accepted when compared with conventional sedated EGD: a multicenter randomized<br />
trial. want <strong>Gastroenterology</strong> to help 125(6), clear pp. 1606–1612. NHS waiting list<br />
backlogs, reduce RTT waiting times and<br />
provide high-quality patient care, get in<br />
touch by calling on 020 3966 9081 or email<br />
Gorelick, A.B., Inadomi, J.M. and Barnett, J.L. 2001. Unsedated small-caliber<br />
esophagogastroduodenoscopy (EGD): less expensive and less time-consuming than<br />
conventional EGD. Journal of Clinical <strong>Gastroenterology</strong> 33(3), pp. 210–214.<br />
Parker, C., Alexandridis, E., Plevris, J., O’Hara, J. and Panter, S. 2016. Transnasal<br />
endoscopy: no gagging no panic! Frontline gastroenterology 7(4), pp. 246–256.<br />
Schuldt, A.-L., Kirsten, H., Tuennemann, J., et al. 2019. Necessity of transnasal<br />
gastroscopy recruitment@18weeksupport.com<br />
in routine diagnostics: a patient-centred requirement analysis. BMJ open<br />
gastroenterology 6(1), p. e000264.<br />
Wellenstein, D.J., Honings, J., Schutte, H.W., et al. 2019. Cost analysis of office-based transnasal<br />
esophagoscopy. European Archives of Oto-Rhino-Laryngology 276(5), pp. 1457–1463.<br />
18 Week Support<br />
www.18weeksupport.com<br />
Dr Matthew Banks Banks<br />
Clinical Lead for <strong>Gastroenterology</strong><br />
18 Week Support<br />
London 3rd Floor, 19-21 Great Tower Street, London EC3R 5AR<br />
Birmingham Unit 25, Lichfield Business Village, The Friary WS13 6QG<br />
GASTROENTEROLOGY TODAY - SPRING 2019
UEG Week – World Class Scientific Research<br />
Meet. Exchange. Evolve<br />
NEWS<br />
UEG Week goes virtual: October 11–13, <strong>2020</strong><br />
ueg.eu/week<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
15
NEWS<br />
Simpler diagnostic process<br />
for adults with suspected<br />
coeliac disease could<br />
reduce NHS waiting lists and<br />
improve patient health faster<br />
The British Society of <strong>Gastroenterology</strong><br />
(BSG) has issued interim guidance,<br />
pending the publication of its new Coeliac<br />
Guidelines in 2021, so that some adults<br />
with suspected coeliac disease can now<br />
be diagnosed based on blood test results<br />
alone, cutting out the long wait for an<br />
endoscopy with biopsy.<br />
Diagnosis of coeliac disease in adults is usually<br />
a two-step process, a blood test to look for<br />
antibodies followed by an endoscopy with<br />
biopsy to look for damage to the intestine.<br />
Early in the coronavirus pandemic, the<br />
BSG recommended that non-emergency<br />
endoscopies should be paused to protect<br />
NHS staff and patients from coronavirus<br />
transmission. This meant that many people with<br />
suspected coeliac disease have been unable to<br />
have an endoscopy as part of their diagnosis.<br />
Hilary Croft, Chief Executive of Coeliac UK<br />
said: “Coeliac UK has previously called for<br />
the national guidelines to review the evidence<br />
for adult no-biopsy diagnosis and so fully<br />
supports the BSG’s new position. This will<br />
enable a greater number of people to gain<br />
a faster diagnosis, without the need to wait<br />
for an endoscopy at the hospital. Getting an<br />
accurate diagnosis of coeliac disease means<br />
keeping gluten in the diet throughout the<br />
testing process - a difficult feat when waiting<br />
lists are long and people feel unwell.”<br />
The interim guidance published due to the<br />
impact of Covid-19 on endoscopy waiting<br />
lists, suggests that a no-biopsy diagnosis can<br />
be used for adults under 55 years of age with<br />
symptoms of coeliac disease who:<br />
• Don’t need an endoscopy to rule out other<br />
conditions<br />
• Have antibody levels (IgA tissue<br />
transglutaminase) at least 10 times the<br />
upper limit of normal<br />
• Have a second positive antibody blood test<br />
(endomysial antibodies (EMA) or tissue<br />
transglutaminase if EMA isn’t available)<br />
A GP can request the initial antibody blood test<br />
but the decision about whether an endoscopy<br />
and biopsy is needed, and the final diagnosis<br />
of coeliac disease, should be made by a<br />
gastroenterologist. The impact of this new<br />
diagnosis pathway will be closely monitored, and<br />
data is being collected to assess the impact of<br />
this new approach for adults. For children, since<br />
2013, guidelines have recommend a no-biopsy<br />
diagnosis for some children.<br />
“These guidelines are good news for those<br />
who meet the criteria for a no-biopsy diagnosis<br />
who will be able to start to feel better sooner<br />
on a gluten free diet, the only treatment for<br />
coeliac disease. However, those that do not<br />
meet the criteria for no-biopsy diagnosis are<br />
likely to face long waiting times as endoscopy<br />
services begin a phased return. Access to<br />
blood tests may still be limited at the moment,<br />
so we encourage people to speak with their<br />
GP for more information about diagnosis<br />
of coeliac disease if they are experiencing<br />
symptoms,” continued Ms Croft.<br />
Coeliac disease is not an allergy or an<br />
intolerance but an autoimmune disease where<br />
the body’s immune system damages the lining<br />
of the small bowel when gluten, a protein<br />
(found in wheat, barley and rye) is eaten.<br />
There is no cure and no medication; the only<br />
treatment is a strict gluten free diet for life. 1<br />
in 100 people in the UK has coeliac disease<br />
but only 30% of those with the condition have<br />
been diagnosed. There are an estimated<br />
half a million people in the UK who have the<br />
condition yet don’t know it.<br />
There is a wide range of symptoms associated<br />
with coeliac disease. Some symptoms may be<br />
confused with irritable bowel syndrome (IBS)<br />
or wheat intolerance, while others may be put<br />
down to stress or getting older.<br />
To make it easier to understand if symptoms<br />
are possibly due to coeliac disease and<br />
discuss further testing with your GP, Coeliac<br />
UK has developed a self assessment test<br />
to make it easier to take that first step to<br />
diagnosis.<br />
Go to www.isitcoeliacdisease.org.uk to take<br />
the online assessment.<br />
WHY NOT WRITE FOR US?<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
<strong>Gastroenterology</strong> <strong>Today</strong> welcomes the submission of<br />
clinical papers and case reports or news that<br />
you feel will be of interest to your colleagues.<br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
16<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com
BIOHIT ULTRA-FAST UFT300<br />
HELICOBACTER PYLORI QUICK TEST<br />
NEWS<br />
A five-minute urease test<br />
for gastric biopsies<br />
As lockdown begins to ease and your endoscopy<br />
services restart, our UFT300 Helicobacter pylori Quick<br />
Test and supporting online training module are more<br />
important than ever.<br />
BIOHIT HealthCare’s quick and simple to use ultra-fast<br />
UFT300 Helicobacter pylori Quick Test is the market-leading<br />
kit for the diagnosis of H. pylori infection in both NHS and<br />
private endoscopy departments. It offers accuracy and a<br />
clinical performance comparable to gold standard methods, 1 Key advantages of the UFT300 Helicobacter pylori Quick Test:<br />
allowing a reliable diagnosis to be made in just five minutes.<br />
This rapid kit provides the opportunity to test, report and • Results in five minutes<br />
treat the patient in a single appointment at the point of<br />
• Ready to use<br />
care – avoiding unnecessary admin and report editing – and • Easy to use<br />
enhances the quality of service.<br />
• High accuracy minimises false positives and negatives<br />
• Direct results during the endoscopy procedure<br />
H. pylori infection is a significant and independent risk factor • Sensitivity 94.3 %, specificity 100 % (compared to histology<br />
for gastric cancer and peptic ulcer disease. It is a primary<br />
and C 13 urea breath test (C 13 UBT n=1000)<br />
cause of chronic gastritis, and leads to atrophy of the gastric • Accuracy 97.5 % compared to histology and C 13 UBT<br />
mucosa if left untreated. The innovative BIOHIT UFT300<br />
• Room temperature storage<br />
Helicobacter pylori Quick Test is a rapid urease test that has<br />
revolutionised the way H. pylori infection is diagnosed and To help users get the most out of the test, BIOHIT offers the<br />
reported in endoscopy. Biopsy specimens from the gastric UFT300 Helicobacter pylori Quick Test online training module.<br />
mucosa can be tested immediately, and a simple colour<br />
This fully interactive training experience is designed for all<br />
change determines the presence of urease – produced by H. endoscopy staff and covers a range of topics, including H.<br />
pylori – within the sample. With extremely high accuracy and pylori infection, urease tests, and the use and interpretation<br />
every result available within five minutes, this ready-to-use of the UFT300 Helicobacter pylori Quick Test. A certificate is<br />
test is the preferred choice for endoscopists.<br />
awarded on completion of the course.<br />
Get in touch with a local representative by contacting: info@biohithealthcare.co.uk<br />
1<br />
Vaira D et al. Accuracy of a new ultrafast rapid urease test to diagnose Helicobacter pylori infection in 1,000 consecutive dyspeptic patients. Aliment Pharmacol Ther 2010; 31, 331-338.<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
BIOHIT HealthCare Ltd<br />
Pioneer House, Pioneer Business Park, North Road,<br />
Ellesmere Port, CHESHIRE, United Kingdom CH65 1AD<br />
Tel. +44 151 550 4 550<br />
info@biohithealthcare.co.uk<br />
www.biohithealthcare.co.uk/endoscopy<br />
17
NEWS<br />
Cost of constipation still<br />
rising in most English<br />
regions, reveals new report<br />
from the independent Bowel<br />
Interest Group<br />
(Letchworth, August <strong>2020</strong>)<br />
Newly released data from the Bowel<br />
Interest Group – published in the <strong>2020</strong><br />
edition of its Cost of Constipation report<br />
– has revealed that the cost of avoidable<br />
emergency admissions to hospital because<br />
of constipation is rising year-on-year in most<br />
regions of England. Just six regions have<br />
seen a drop in the cost and/or number of<br />
admissions for constipation compared to two<br />
years prior. This comes at a time when the<br />
NHS is already under stress and is dealing<br />
with the backlog of patients with chronic<br />
conditions who have had their treatments<br />
delayed because of the coronavirus<br />
pandemic.<br />
The Cost of Constipation report reveals the<br />
impact that constipation has on patients’<br />
quality of life, the significant cost of<br />
constipation to the NHS as well as how this<br />
varies by region. Nationally, the cost per<br />
100,000 population of avoidable constipationrelated<br />
emergency admissions was over<br />
£158,000 in 2018/19. This represents a<br />
15% rise compared with 2016/17 (around<br />
£137,000). Regional variations were<br />
marked, ranging from around £106,000 per<br />
100,000 in Bristol, North Somerset & South<br />
Gloucestershire, through to £244,000 per<br />
100,000 in Humber, Coast & Vale. This level<br />
of variation underlines the importance of<br />
establishing and implementing best practice<br />
bowel management across the country.<br />
At a national level, the report shows that<br />
poor bowel health and chronic constipation,<br />
which are debilitating for hundreds and<br />
thousands of people in the UK, cost the NHS<br />
£81 million per year in admissions to A&E for<br />
constipation. This cost is likely to be much<br />
higher when GP visits, home visits and over<br />
the counter laxatives are taken into account.<br />
Other key figures include:<br />
• £168 million was spent treating<br />
constipation in 2018/19. This includes<br />
avoidable admissions to A&E for<br />
constipation (£81 million) and prescription<br />
laxative costs (£87 million).<br />
• The cost of treating constipation in 2018/19<br />
is equivalent to funding 7304 newlyqualified<br />
nurses for a year.<br />
• Only 6 out of 42 regions (STPs or ICSs<br />
as applicable) in England have seen a<br />
decrease in the number and/or cost of<br />
avoidable emergency admissions for<br />
constipation.<br />
Some leading NHS Trusts in England have<br />
established formal Bowel Management<br />
Pathways and these pioneering initiatives<br />
are starting to offer empirical proof of their<br />
value, both in transforming patients’ lives<br />
and reducing the cost burden on the NHS.<br />
The Bowel Interest Group publicises clinical<br />
best practice on its website, and further<br />
information from the National Institute for<br />
Health and Care Excellence also offers further<br />
guidance for practitioners[1].<br />
Dr Ben Disney, Consultant Gastroenterologist<br />
at Coventry and Warwickshire University<br />
Hospitals Trust and Bowel Interest Group<br />
board member, comments, “This latest<br />
output from the Bowel Interest Group should<br />
make everyone sit up and take notice. Not<br />
only does chronic constipation ruin people’s<br />
lives, it also is causing the NHS unnecessary<br />
costs, largely because dedicated Bowel<br />
Management Pathways are not yet standard<br />
best practice. Pioneering work in this area has<br />
clearly shown a strong return on investment<br />
from such pathways, both in terms of patient<br />
outcomes and cost reduction. At a time when<br />
POSTER SUBMISSIONS<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
If you have submitted a poster to previous BSG or<br />
ENDOLIVE events and would like it published in<br />
<strong>Gastroenterology</strong> <strong>Today</strong> please forward a PDF of your<br />
poster to the email address listed below.<br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com<br />
18
NEWS<br />
our NHS is under such pressure, failing to<br />
establish these pathways would seem poor<br />
practice. Modern healthcare is not simply<br />
about treating the escalating rise in chronic<br />
conditions, but also taking pre-emptive action<br />
to create more ‘well societies’. Effective bowel<br />
management is just one of the initiatives<br />
that help foster healthier populations that<br />
consume less healthcare.”<br />
The Bowel Interest Group is an independent<br />
multidisciplinary organisation dedicated to<br />
improving bowel care for patients.<br />
You can download the full report free of<br />
charge by visiting:<br />
https://bowelinterestgroup.co.uk/<br />
resources/cost-of-constipationreport-<strong>2020</strong>/<br />
New information pack<br />
supports GPs in best practice<br />
treatment of constipation<br />
(Letchworth, February <strong>2020</strong>) Independent<br />
clinical and patient organisation, The Bowel<br />
Interest Group (BIG), has published a new<br />
information pack for General Practices on<br />
managing acute constipation through key<br />
therapeutic stages, ensuring that patients<br />
are not left for long periods with treatments<br />
that are not working.<br />
Constipation (and its frequent companion<br />
symptom – faecal incontinence) ruins lives.<br />
Yet the condition is still under-managed in<br />
the National Health Service, despite some<br />
fundamentally important foundation work 1 .<br />
In particular, survey work with GPs,<br />
conducted by BIG in 2019, has revealed<br />
that supporting materials for healthcare<br />
professionals at the primary level on<br />
constipation management are scant, and<br />
are one of the key resources sought by<br />
GPs. Most of these patients are treated<br />
empirically with laxatives, with little subtlety<br />
of which agent suits the individual patient’s<br />
symptoms. Respondents to the survey said<br />
they would welcome useful resources on<br />
the issue. This new document is one step in<br />
redressing that balance.<br />
Poor bowel health and chronic constipation<br />
is debilitating for hundreds and thousands of<br />
people in the UK. In 2017/18, it cost the NHS<br />
£162 million in constipation treatment, of<br />
which £71 million was caused by unplanned,<br />
avoidable emergency admissions, and<br />
£91 million by spending on prescription<br />
laxatives i .<br />
The newly published information pack<br />
- Dealing with Acute Constipation,<br />
Information for General Practitioners -<br />
summarises key research on constipation<br />
and treatment options and combines them<br />
into a simple diagram to help provide a<br />
best practice pathway for general practice<br />
in its recognition, treatment and point of<br />
escalation of the available therapies for<br />
acute constipation.<br />
Professor Anton Emmanuel, Consultant<br />
Gastroenterologist at UCLH and the National<br />
Hospital for Neurology & Neurosurgery,<br />
lead the compilation of the new information<br />
pack. He notes, “Many Trusts have now<br />
created, or are developing, dedicated bowel<br />
management pathways based on NICE<br />
guidance, and are already experiencing the<br />
resulting improved patient outcomes. BIG<br />
have created a management pathway based<br />
on the NICE Clinical Knowledge Summary.<br />
This document is aimed at all clinicians,<br />
specialist care professionals, general<br />
practitioners and commissioners to help<br />
understand the rationale and positioning<br />
of this therapy that can have a profoundly<br />
positive effect on people’s health, quality of<br />
life, dignity and requirement for healthcare.”<br />
i<br />
Bowel Independence Group, The Cost of<br />
Constipation Report 2019<br />
WHY NOT WRITE FOR US?<br />
<strong>Gastroenterology</strong> <strong>Today</strong> welcomes the submission of<br />
clinical papers and case reports or news that<br />
you feel will be of interest to your colleagues.<br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
19
NEWS<br />
UEG Week Goes Virtual!<br />
UEG Week Virtual <strong>2020</strong>, October 11-13, <strong>2020</strong><br />
With over 13,000 attendees from more than<br />
120 countries in 2019, UEG Week is one of<br />
the world’s largest and most prestigious<br />
digestive health meetings. Given the<br />
present circumstances facing the world<br />
today, the decision has been made to hold<br />
UEG Week virtually this October, ensuring<br />
the safety of the digestive health community<br />
while still allowing advanced scientific<br />
exchange.<br />
3-day schedule, with state-of-the-art science<br />
continuing to form an essential part of the<br />
meeting and abstract review process. As well<br />
as accessing brand new research via latebreaking<br />
abstracts, delegates will also be able<br />
to explore the status and impact of COVID-19 on<br />
gastrointestinal and hepatology-related fields.<br />
The live programme will run from 08:30-<br />
20:30 CET each day of the congress. For<br />
those accessing the meeting from different<br />
time zones, the UEG Week platform will be<br />
accessible 24 hours a day to browse ondemand<br />
content. The virtual platform will also<br />
remain accessible after the live event, until the<br />
end of December <strong>2020</strong>, and all recordings<br />
will then be permanently available in the UEG<br />
Library.<br />
The majority of the programme features live<br />
interaction between moderators, speakers<br />
and the audience, providing the opportunity<br />
for debate and knowledge exchange. Each<br />
day, there will also be a live broadcast from the<br />
UEG Week Virtual <strong>2020</strong> TV Studio. Featuring<br />
the most exciting and newsworthy topics<br />
from the congress, participants can tune into<br />
the studio and ask real-time questions via a<br />
specialised Q&A virtual tool. This studio will<br />
also host the hugely popular ‘Mistakes in…’<br />
sessions, featuring a range of topics including<br />
pancreatitis, alcohol-related liver disease,<br />
small bowel bleeding and chronic diarrhoea.<br />
Practical-minded delegates can benefit<br />
from the live streamed endoscopy and live<br />
ultrasonography demonstrations, providing<br />
a unique opportunity for attendees to watch<br />
and learn techniques from some of the world’s<br />
leading specialists.<br />
As for postgraduate teaching, UEG is pleased<br />
to offer a ‘best of’ Postgraduate Teaching<br />
programme from previous congresses. Taking<br />
place virtually between November 27-28 <strong>2020</strong>,<br />
this two-day event will provide attendees with<br />
the most important knowledge in digestive<br />
health from renowned experts, in a highly<br />
interactive format. Registration for this event<br />
opens on August 10, <strong>2020</strong>.<br />
“We remain dedicated to organising a highquality<br />
meeting and our programme will still<br />
deliver the latest and greatest in science”,<br />
adds Axel Dignass. “Now more accessible<br />
to our community throughout the world, we<br />
are thrilled to bring delegates this new virtual<br />
platform and I am thoroughly looking forward<br />
to welcoming new and returning delegates to<br />
UEG Week Virtual <strong>2020</strong>.”<br />
Visit ueg.eu/week<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
Axel Dignass, UEG President, explains,<br />
“COVID-19 is the greatest global health<br />
challenge we have seen in decades. For UEG,<br />
the health and safety of our community is a<br />
top priority. We now lead by example in taking<br />
this important decision and invite attendees<br />
to meet, exchange and evolve virtually for the<br />
best gastroenterology congress in the world.”<br />
As always, the UEG Scientific Committee has<br />
pieced together the congress programme,<br />
featuring a variety of exciting topics covering<br />
all aspects of digestive health. A range of<br />
session types will exist to showcase the best<br />
science across these areas, ensuring the<br />
delivery of a first-class and multidisciplinary<br />
programme to all attendees, no matter where<br />
they will be joining the congress from.<br />
The programme has been adapted to a new<br />
20
NEWS<br />
Bowel Interest Group<br />
launches updated Interactive<br />
Treatment Pathway for<br />
chronic constipation patients<br />
(Letchworth, 18 June <strong>2020</strong>)<br />
Research has shown that chronic<br />
constipation is costing the NHS £71 million/<br />
year in avoidable, unplanned emergency<br />
hospital admissions. Bowel complaints carry<br />
an enormous stigma, with one on five too<br />
embarrassed to talk to their GP – the same<br />
level of embarrassment associated with<br />
erectile dysfunction. Even more importantly,<br />
there is a long-term impact on wellbeing<br />
and quality of life. Chronic constipation can<br />
cause debilitating physical and psychological<br />
distress, especially as it can cause other<br />
issues, such as chronic pain and urinary<br />
tract infections (UTIs). Yet constipation is<br />
a treatable and manageable condition,<br />
so earlier and improved treatment would<br />
alleviate an unnecessary burden on the NHS.<br />
Data shows that lack of information and<br />
dedicated bowel management pathways is<br />
impeding the early escalation of chronic<br />
constipation towards effective treatment<br />
and improved patient outcomes. To assist<br />
healthcare professionals in both primary<br />
and acute sectors, the Bowel Interest<br />
Group has launched an updated edition<br />
of its Interactive Treatment Pathway for<br />
chronic constipation.<br />
The pathway constitutes an easy reference<br />
guide for treating adults with chronic<br />
constipation. The interactive treatment<br />
pathway starts at the initial consultation<br />
through to third line therapies and when<br />
to refer to secondary care. It has been<br />
specifically developed to improve care<br />
and reduce costs associated with chronic<br />
constipation in the community.<br />
Recognising that this issue is a particularly<br />
significant problem in primary care, the<br />
guidance is structured pragmatically to<br />
allow quick and safe decision making. The<br />
first appointment may just cover history and<br />
examination. The pathway would then assist<br />
by standardising the lifestyle measures<br />
which have evidence to support them. The<br />
pathway has been developed from the<br />
NICE CKS on constipation and is interactive<br />
to allow healthcare professionals to click<br />
through to the relevant section during<br />
patient consultation.<br />
Professor Anton Emmanuel, Consultant<br />
Gastroenterologist at UCLH and the<br />
National Hospital for Neurology &<br />
Neurosurgery, who led the development of<br />
the Interactive Pathway, comments:<br />
“Despite the availability of specific NICE<br />
guidance on bowel management, and the<br />
pioneering work of some NHS Trusts on<br />
the issue, widespread effective treatment<br />
of chronic constipation still has some way<br />
to go. It is therefore important that BIG<br />
have published this Interactive Treatment<br />
Pathway which simply and safely leads<br />
practitioners through the clinical decision<br />
making process, based on the NICE<br />
guidance. Better treatment of constipation<br />
reduces the burden on the NHS while also<br />
having a profoundly positive effect on<br />
people’s health, quality of life, dignity and<br />
requirement for healthcare. This document<br />
is a support tool aimed at all clinicians,<br />
specialist care professionals, general<br />
practitioners and commissioners and can<br />
be used in tandem with the other important<br />
information on the subject published by the<br />
Bowel Interest Group.”<br />
WHY NOT WRITE FOR US?<br />
<strong>Gastroenterology</strong> <strong>Today</strong> welcomes the submission of<br />
clinical papers and case reports or news that<br />
you feel will be of interest to your colleagues.<br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
21
COMPANY NEWS<br />
BIOHIT SUPPLIES COVID-19<br />
DETECTION KITS IN THE UK<br />
BIOHIT Healthcare Ltd is now distributing test kits for the<br />
diagnosis of both current and past COVID-19 infections to<br />
help in the fight against coronavirus in the UK. The new<br />
product line includes the MutaPLEX ® Coronavirus kit from<br />
Immundiagnostik AG (IDK) – a real-time RT-PCR assay to<br />
screen for infected individuals – and Epitope Diagnostics Inc’s<br />
(EDI’s) immunodiagnostic tests for IgM and IgG COVID-19<br />
antibodies, to detect past infections.<br />
The IDK MutaPLEX coronavirus screening assay allows the detection of<br />
SARS-CoV-2 viral RNA in a variety of biological specimens, especially nasal/<br />
throat swabs. This real time RT-PCR kit contains all the reagents, primers<br />
and dual-labelled probes required for the amplification and simultaneous<br />
differentiation of RNA from SARS-CoV-2 and other betacoronaviruses, as<br />
well as house-keeping genes designed to prevent false negative results<br />
due to insufficient sample collection or transport problems.<br />
EDI’s Novel Coronavirus COVID-19 ELISA kits provide qualitative detection<br />
of antibodies in patient serum, indicating a past COVID-19 infection. The<br />
IgM assay provides the earliest immunodiagnostic indication of an infection,<br />
while the IgG test can be used to aid detection and provide an indication<br />
of long-term immunological response, making it particularly useful in cases<br />
where clustering is suspected or differential diagnosis is required.<br />
These tests extend and complement BIOHIT’s repertoire of diagnostic kits<br />
for gastroenterology, aiding the evaluation of patients with both GI and<br />
upper respiratory complaints, as COVID-19 may include stomach and<br />
bowel symptoms in some cases. Inflammatory bowel disease patients<br />
being treated with immunosuppressive agents should also be considered<br />
at high risk for COVID-19, making differential diagnosis essential.<br />
Graham Johnson, Managing Director of BIOHIT Healthcare Ltd,<br />
commented: “Access to diagnostic testing is vital to coordinate an<br />
effective response to this global pandemic. By distributing these kits,<br />
we hope to streamline and simplify the UK supply chain, allowing more<br />
people to be tested sooner. This is just one piece of the puzzle, but it is<br />
important that we all do our small part in protecting both individuals and<br />
the NHS and, ultimately, saving lives.”<br />
About BIOHIT Healthcare Ltd<br />
BIOHIT Healthcare Ltd (www.biohithealthcare.co.uk) is part of<br />
the Finnish public company, BIOHIT OYJ, which specialises in the<br />
development, manufacture and marketing of products and analysis<br />
systems for the early diagnosis and prevention of gastrointestinal<br />
diseases. The company’s many unique and patented diagnostic<br />
tests transform clinical practice and make screening, diagnosis and<br />
monitoring of gastrointestinal diseases efficient and cost effective.<br />
Non-invasive diagnostics are at the core of BIOHIT’s offering, making<br />
it the provider of choice for leading gastroenterologists and laboratory<br />
scientists worldwide.<br />
POSTER SUBMISSIONS<br />
GASTROENTEROLOGY TODAY - AUTUMN <strong>2020</strong><br />
22<br />
If you have submitted a poster to previous BSG or<br />
ENDOLIVE events and would like it published in<br />
<strong>Gastroenterology</strong> <strong>Today</strong> please forward a PDF of your<br />
poster to the email address listed below.<br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com
A Huge Thank You to The NHS from the<br />
<strong>Gastroenterology</strong> <strong>Today</strong> Junior Team<br />
Carys - Age 6<br />
Zoe - Age 7<br />
Luke - Age 10<br />
Joseph - Age 5<br />
Thank You<br />
Thank You<br />
Thank You<br />
Aoife - Age 4<br />
Charlotte - Age 3<br />
Rory - Age 2
Helicobacter Test INFAI ®<br />
The most used 13 C-urea breath test for the<br />
diagnosis of Hp-infection worldwide<br />
• more than 4.5 million INFAI tests performed in Europe<br />
• approved for children from the ages of 3 to 11<br />
• special INFAI test for patients with dyspepsia taking PPIs<br />
• cost-effective CliniPac Basic version for hospital use<br />
INFAI Institute for Biomedical Analysis & NMR Imaging, INFAI UK Ltd<br />
Innovation Centre, University Science Park, University Road, Heslington, YORK YO10 5DG, UK<br />
Phone +44 1904 435 228 - Fax +44 1904 435 229 - mail: info@infai.co.uk - Visit us at www.infai.com