You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Volume 34 No. 5 <strong>Spring</strong> <strong>2024</strong>
CONTENTS<br />
CONTENTS<br />
<strong>Gastroenterology</strong> <strong>Today</strong><br />
Developed to support patient<br />
acceptance 1–3<br />
Shape<br />
• 89.5% of patients found it easy to administer<br />
a torpedo-shaped 1g mesalazine suppository *3,4<br />
* This study was not conducted with Octasa ® 1g suppositories.<br />
Support<br />
• Simple, visual patient materials available to<br />
promote acceptance and adherence 2<br />
Once daily<br />
• For the induction and maintenance of remission<br />
of mild to moderate ulcerative proctitis 1<br />
4 EDITOR’S COMMENT<br />
7 FEATURE The impact of endoscopist performance and patient<br />
factors on distal adenoma detection and colorectal<br />
cancer incidence<br />
21 FEATURE The cost of illness analysis of inflammatory<br />
bowel disease<br />
31 COMPANY NEWS<br />
This issue edited by:<br />
Aaron Bhakta<br />
c/o Media Publishing Company<br />
Greenoaks, Lockhill<br />
Upper Sapey, Worcester, WR6 6XR<br />
ADVERTISING & CIRCULATION:<br />
Media Publishing Company<br />
Greenoaks, Lockhill<br />
Upper Sapey, Worcester, WR6 6XR<br />
Tel: 01886 853715<br />
E: info@mediapublishingcompany.com<br />
www.ambulanceukonline.com<br />
PUBLISHED DATES:<br />
March, June, September and December.<br />
COPYRIGHT:<br />
Media Publishing Company<br />
Greenoaks<br />
Lockhill<br />
Upper Sapey, Worcester, WR6 6XR<br />
Access the Octasa ® 1g suppositories<br />
support materials for your patients<br />
at tillotts.co.uk<br />
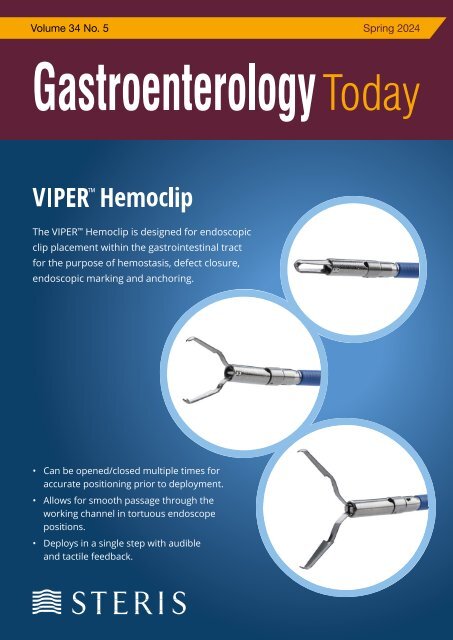
COVER STORY<br />
The Viper Hemoclip is designed for endoscopic clip placement within the<br />
gastrointestinal tract for the purpose of hemostasis, defect closure, endoscopic<br />
marking and anchoring.<br />
• Available in 5 sizes (9, 11, 13, 16, 18mm opening)<br />
PUBLISHERS STATEMENT:<br />
The views and opinions expressed in<br />
this issue are not necessarily those of<br />
the Publisher, the Editors or Media<br />
Publishing Company<br />
Next Issue Summer <strong>2024</strong><br />
Designed in the UK by TGDH<br />
• 7mm tail length for visibility<br />
OCTASA 1g Suppositories (mesalazine) - Prescribing Information<br />
Presentation: Suppository containing 1g mesalazine. Indications: Treatment<br />
of acute mild to moderate ulcerative proctitis. Maintenance of remission of<br />
ulcerative proctitis Dosage and administration: Adults and older people:<br />
Acute treatment - one Octasa 1 g Suppository once daily (equivalent to 1<br />
g mesalazine daily) inserted into the rectum. Maintenance treatment - one<br />
Octasa 1 g Suppository once daily (equivalent to 1 g mesalazine daily) inserted<br />
into the rectum. Children: Limited experience and data for use in children.<br />
Method of administration: for rectal use, preferably at bedtime. Duration of<br />
use to be determined by the physician. Contra-indications: Hypersensitivity<br />
to salicylates or any of the excipients, severe impairment of hepatic or renal<br />
function. Warnings and Precautions: Blood tests and urinary status (dip<br />
sticks) should be determined prior to and during treatment, at discretion of<br />
treating physician. Caution in patients with impaired hepatic function. Do not<br />
use in patients with impaired renal function. Consider renal toxicity if renal<br />
function deteriorates during treatment. Cases of nephrolithiasis have been<br />
reported with mesalazine treatment. Ensure adequate fluid intake during<br />
treatment. Monitor patients with pulmonary disease, in particular asthma, very<br />
carefully. Patients with a history of adverse drug reactions to sulphasalazine<br />
should be kept under close medical surveillance on commencement of<br />
therapy, discontinue immediately if acute intolerance reactions occur (e.g.<br />
abdominal cramps, acute abdominal pain, fever, severe headache and rash).<br />
Severe cutaneous adverse reactions (SCARS), including Drug reaction with<br />
eosinophilia and systemic symptoms (DRESS), Stevens-Johnson Syndrome<br />
(SJS) and toxic epidermal necrolysis (TEN) have been reported. Stop treatment<br />
immediately if signs and symptoms of severe skin reactions are seen.<br />
Mesalazine may produce red-brown urine discoloration after contact with<br />
sodium hypochlorite bleach (e.g. in toilets cleaned with sodium hypochlorite<br />
contained in certain bleaches). Interactions: No interaction studies have<br />
been performed. May increase the myelosuppressive effects of azathioprine,<br />
6-mercaptopurine or thioguanine. May decrease the anticoagulant activity<br />
of warfarin. Fertility, pregnancy and lactation: Only to be used during<br />
pregnancy and lactation when the potential benefit outweighs the possible<br />
risk. No effects on fertility have been observed. Adverse reactions: Rare:<br />
Headache, dizziness, myocarditis, pericarditis, abdominal pain, diarrhoea,<br />
flatulence, nausea, vomiting, constipation, photosensitivity, Very rare: Altered<br />
blood counts (aplastic anaemia, agranulocytosis, pancytopenia, neutropenia,<br />
leukopenia, thrombocytopenia), peripheral neuropathy, allergic and fibrotic<br />
lung reactions (including dyspnoea, cough, bronchospasm, alveolitis,<br />
pulmonary eosinophilia, lung infiltration, pneumonitis), acute pancreatitis,<br />
impairment of renal function including acute and chronic interstitial nephritis<br />
and renal insufficiency, alopecia, myalgia, arthraligia, hypersensitivity<br />
reactions (such as allergic exanthema, drug fever, lupus erythematosus<br />
syndrome, pancolitis), changes in liver function parameters (increase in<br />
transaminases and parameters of cholestasis), hepatitis, cholestatic hepatitis,<br />
oligospermia (reversible). Not known: Nephrolithiasis, Drug reaction with<br />
eosinophilia and systemic symptoms, Stevens-Johnson syndrome, and toxic<br />
epidermal necrolysis. Consult the Summary of Product Characteristics in<br />
relation to other adverse reactions. Marketing Authorisation Numbers,<br />
Package Quantities and basic NHS price: PL36633/0011; packs of 10<br />
suppositories (£9.87) and 30 suppositories (£29.62). Legal category: POM.<br />
Marketing Authorisation Holder: Tillotts Pharma UK Ltd, The Larbourne<br />
Suite, The Stables, Wellingore Hall, Wellingore, Lincolnshire, LN5 0HX, UK.<br />
Octasa is a trademark. © 2021 Tillotts Pharma UK Ltd. Further Information<br />
is available from the Marketing Authorisation Holder. Date of preparation of<br />
PI: November 2022<br />
Adverse events should be reported.<br />
Reporting forms and information can be found at<br />
https://yellowcard.mhra.gov.uk. Adverse events<br />
should also be reported to Tillotts Pharma UK Ltd.<br />
(address as above) Tel: 01522 813500.<br />
References<br />
1. Octasa ® 1g Suppositories – Summary of Product Characteristics.<br />
2. Ghosh S, Daperno M. <strong>Gastroenterology</strong> 2015; 148(4): 701–704.<br />
3. Andus T et al. Inflamm Bowel Dis 2010; 16(11): 1947–1956.<br />
4. Data on file. Tillotts Pharma UK Limited. [Pharmaceutical<br />
Development Information: Octasa ® 1g suppositories] – January 2022.<br />
Date of preparation: December 2022. PU-00987.<br />
Journal: <strong>Gastroenterology</strong> <strong>Today</strong> Tillotts: Octasa Range Ad Job no: 04994<br />
Size: 297 x 210 mm Bleed: 3 mm Supply as: HR PDF<br />
• Passes through retroflexed scopes and duodenoscope<br />
How the Assurance Clip Works and Affects Patient Flow and Outcomes<br />
Hemostatic clips are suggested for the treatment of gastrointestinal bleeding and have<br />
been shown to be more effective for the treatment of GI bleeding than other modalities,<br />
such as epinephrine injection alone. 1 Prophylactic endoscopic clip closure of large<br />
mucosal defects following polyp resection has also been demonstrated to reduce the risk<br />
of post procedure bleeding. 2,3<br />
The Assurance hemostatic clip offers 360-degree, one-to-one rotation, ability to reposition<br />
the clip multiple times prior to deployment, opening widths of 9, 11, 13, 16, and 18<br />
millimeters and a retained clip length of 12.5 - 14.5mm millimeters.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
3
EDITOR’S COMMENT<br />
EDITOR’S COMMENT<br />
BMA v Government<br />
“After 5 years<br />
the system<br />
has managed<br />
to produce<br />
a cohort of<br />
intelligent,<br />
qualified and<br />
motivated<br />
doctors who<br />
within a very<br />
short timescale<br />
are fed up”<br />
Something has gone very wrong in medical undergraduate and postgraduate training. Anyone involved in<br />
medical school admissions or with a friend or family member applying for medical school will be aware of<br />
the hoops that need to jumped through, the numbers wanting to study medicine and the competitive nature<br />
of the entire process. And yet, after 5 years the system has managed to produce a cohort of intelligent,<br />
qualified and motivated doctors who within a very short timescale are fed up, willing to strike and looking to<br />
leave medicine or the UK.<br />
The media reports suggest this all boils down to pay but it is clearly much more complex. There are<br />
significant financial issues which start on day one at medical school. New students can only dream of no<br />
fees, student grants, housing benefit and 12 months accommodation provided on qualification. The erosion<br />
of all of these feed into the pay issue.<br />
When qualifying with huge debt all future expenses come under the spotlight: post graduate professional<br />
exams, GMC fees and indemnity fees to name but a few. None of these are optional.<br />
Aspiration to become a senior doctor is further dampened by the ongoing negotiations between the BMA<br />
and government on the consultant contract, a pension scheme that has become so complex barely anyone<br />
seems to truly understand it and primary care under more pressure than ever before.<br />
The solution has to be more than just a pay rise. Unless all aspects of the costs associated with<br />
undergraduate and postgraduate medicine are addressed any single solution is likely to be a temporary<br />
band aid.<br />
A Poullis<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
Publishers Comment<br />
On behalf of everyone involved with the publishing of <strong>Gastroenterology</strong> <strong>Today</strong> I would like to say a big<br />
thank you to our contributors for their input and a special thank you to our advertisers as without their<br />
ongoing support we would not be able to print and despatch copies of this very unique publication to all<br />
<strong>Gastroenterology</strong> Departments and Endoscopy Units. Wishing you all a prosperous <strong>2024</strong>.<br />
Terry Gardner<br />
Publisher<br />
4
FEATURE<br />
EyeMAX<br />
Enhanced Clarity,<br />
Improved Diagnoses<br />
THE IMPACT OF ENDOSCOPIST<br />
PERFORMANCE AND PATIENT FACTORS<br />
ON DISTAL ADENOMA DETECTION AND<br />
COLORECTAL CANCER INCIDENCE<br />
Sharon Power 1* , Kate Wooldrage 1 , Brian P. Saunders 2,3 and Amanda J. Cross 1<br />
Power et al. BMC <strong>Gastroenterology</strong> (<strong>2024</strong>) 24:44 https://doi.org/10.1186/s12876-024-03125-x<br />
RESEARCH<br />
Abstract<br />
Background High quality endoscopy is key for detecting and removing<br />
precursor lesions to colorectal cancer (CRC). Adenoma detection<br />
rates (ADRs) measure endoscopist performance. Improving other<br />
components of examinations could increase adenoma detection.<br />
Introduction<br />
Colorectal cancer (CRC) is the fourth most common cancer with over<br />
42,000 cases diagnosed in the UK annually [1]. Effective screening for<br />
CRC enables the removal of precursor lesions, preventing CRC, and<br />
the detection of CRC at an earlier stage, significantly improving patient<br />
outcomes [2, 3].<br />
Experience Superior<br />
Image Quality<br />
120° Angle of HD Vision<br />
Aims To investigate how endoscopist performance at flexible<br />
sigmoidoscopy (FS) affects adenoma detection and CRC incidence.<br />
Methods Among 34,139 participants receiving FS screening by the<br />
main endoscopist at one of 13 centres in the UK FS Screening Trial,<br />
median follow-up was 17 years. Factors examined included family<br />
history of CRC, bowel preparation quality, insertion and withdrawal<br />
time, bowel segment reached, patient pain and ADR. Odds ratios (OR)<br />
for distal adenoma detection were estimated by logistic regression.<br />
Hazard ratios (HR) for distal CRC incidence were estimated by<br />
Cox regression.<br />
Flexible sigmoidoscopy (FS) involves inserting a thin tube into<br />
the rectum to visualise ~ 60 cm of the distal colorectum [4]. FS<br />
screening reduces CRC incidence and mortality [5–8]; in the UK<br />
Flexible Sigmoidoscopy Screening Trial (UKFSST), CRC incidence<br />
and mortality was reduced by 35% and 41%, respectively, in those<br />
screened compared to controls [9].<br />
Endoscopic examination accuracy is dependent on endoscopist skill<br />
and experience, with higher quality exams associated with better<br />
patient outcomes [10]. Adenoma detection rates (ADRs) are used to<br />
assess endoscopist performance [11]. Low ADRs are associated with<br />
Your Trusted Partner<br />
in Endoscopy<br />
020 8016 1990<br />
sales@micro-tech-uk.com<br />
www.micro-tech-uk.com<br />
Powerful, Intuitive Lightning<br />
Total Control 4 Way Agulation<br />
Scan to Find Out More<br />
Results At screening, 4,104 participants had distal adenomas<br />
detected and 168 participants developed distal CRC during follow-up.<br />
In multivariable models, a family history of CRC (yes vs. no: OR 1.40,<br />
95%CI 1.21–1.62), good or adequate bowel preparation quality (vs.<br />
excellent: OR 0.84, 95%CI 0.74–0.95; OR 0.56, 95%CI 0.49–0.65,<br />
respectively) and longer insertion and withdrawal times (≥ 4.00 vs. <<br />
2.00 min: OR 1.96, 95%CI 1.68–2.29; OR 32.79, 95%CI 28.22– 38.11,<br />
respectively) were associated with adenoma detection. Being screened<br />
by endoscopists with low or intermediate ADRs, compared to high<br />
ADRs, was positively associated with CRC incidence (multivariable:<br />
HR 4.71, 95%CI 2.65–8.38; HR 2.16, 95%CI 1.22–3.81, respectively).<br />
Conclusions Bowel preparation quality and longer insertion and<br />
withdrawal time are key for improving distal adenoma detection. Higher<br />
ADRs were associated with a lower risk of distal CRC.<br />
Keywords Colorectal cancer, Endoscopic screening, Adenoma<br />
detection, Key performance indicators, Flexible sigmoidoscopy<br />
*Correspondence:<br />
Sharon Power<br />
s.power18@imperial.ac.uk<br />
Full list of author information is available at the end of the article<br />
higher rates of interval CRCs [12] and post-colonoscopy CRC mortality<br />
[13]. Large variability exists in ADRs between endoscopists [14–16],<br />
with quality of bowel preparation [17, 18], depth of endoscope insertion,<br />
segment of bowel reached and withdrawal time all related to ADRs [14].<br />
Higher quality withdrawal techniques are associated with lower miss<br />
rates for adenomas [19].<br />
The Joint Advisory Group on gastrointestinal endoscopy, the British<br />
Society of <strong>Gastroenterology</strong> and the Association of Coloproctology of<br />
Great Britain and Ireland have developed key performance indicators<br />
(KPIs) for endoscopy, which include ADRs, bowel preparation quality,<br />
withdrawal time, comfort and completeness of examination [20]. These<br />
KPIs are accompanied by quality assurance measures, which provide<br />
minimal standards and aspirational targets for endoscopists [20].<br />
However, there is a lack of data on KPIs and long-term outcomes. The<br />
UKFSST offers the opportunity to examine KPIs in relation to adenoma<br />
detection and distal CRC incidence.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
7
FEATURE<br />
Power et al. BMC <strong>Gastroenterology</strong> (<strong>2024</strong>) 24:44<br />
Page 3 of 13<br />
FEATURE<br />
Methods<br />
Study design<br />
Between November 1994 and March 1999, the UKFSST recruited men<br />
and women aged 55–64 years from general practices serving 14 UK<br />
hospitals; details reported previously [9]. Adenoma incidence increases<br />
after the age of 50 years but levels out before 60 years [15, 21];<br />
screening around 60 years of age offered the optimum opportunity to<br />
detect adenomas [21]. Participants were excluded if they were unable<br />
to provide consent; had a history of CRC, adenomas or inflammatory<br />
bowel disease; had severe/terminal disease, life expectancy of < 5<br />
years, or a sigmoidoscopy/colonoscopy within the previous 3 years.<br />
Eligible individuals were randomised to either the intervention (n =<br />
57,237, invitation to once-only FS screening), or control arm (n =<br />
113,195, no screening and no further contact) (Fig. 1).<br />
UKFSST endoscopists were previously ranked by their ADR (estimated<br />
as the proportion of participants that had ≥ 1 distal adenoma detected)<br />
into high-, intermediate-, or low-detectors, with corresponding<br />
ADRs of 15%, 12% and 9%, respectively [15]; these groups were<br />
used in this analysis. The order in which participants were screened<br />
revealed a learning effect for the endoscopists’ ADR [15]; thus, we<br />
created a variable that grouped participants according to the order of<br />
examination occurrence: the first 500 participants examined by each<br />
endoscopist and those examined later.<br />
Outcome ascertainment<br />
Information on date, site and morphology of cancers and date of<br />
emigrations and deaths were collected from National cancer registries,<br />
the National Health Service (NHS) Central Register, National Services<br />
Scotland, NHS Digital and the Office for National Statistics.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
We excluded those who died or were diagnosed with CRC prerandomisation,<br />
those in a family history study receiving colonoscopy<br />
screening, those screened by an endoscopist other than the main<br />
endoscopist at each centre, those in the pilot centre, those screened<br />
within the first two months at one centre where the pathologist was<br />
over-diagnosing adenomas, those diagnosed with CRC at baseline<br />
and those with incomplete exams (Fig. 1). Of the 34,139 participants<br />
remaining, 1,810 received multiple FS examinations (89% repeated due<br />
to poor bowel preparation quality; Supplementary Table 1). Only one<br />
exam per participant was included in the analysis; if FS was repeated<br />
due to poor bowel preparation, the last complete exam was included,<br />
but if FS was repeated for other reasons, the earliest complete exam<br />
was used. If any exams had polyps detected, these rules were applied<br />
within exams with polyps only.<br />
Endoscopists were registrar-level gastroenterologists/surgeons with<br />
3–8 years of experience post-basic medical qualification and must<br />
have performed a minimum of 50 supervised and 100 unsupervised<br />
endoscopies [15]. Participants were to administer a single phosphate<br />
enema (Fletchers’ phosphate enema; Forest Laboratories UK Ltd.,<br />
Bexley, Kent), one hour before leaving home for their examination<br />
[15]. Sedation was not routinely used during FS examination [15]. All<br />
endoscopists were to advance the scope (60 cm Olympus videoendoscope<br />
(CF-200S)) as far as possible without causing undue<br />
discomfort (normally to the sigmoid colon/descending colon junction)<br />
and to remove polyps < 10 mm, leaving intact polyps < 3 mm deemed<br />
to be hyperplastic in the distal 4 cm of the rectum [15]. Follow-up<br />
colonoscopy was arranged for participants at high risk (≥ 3 adenomas,<br />
a polyp ≥ 10 mm, an adenoma with villous/tubulovillous histology, or<br />
high-grade dysplasia, malignant disease, or ≥ 20 hyperplastic polyps<br />
above the distal rectum) [15].<br />
Exposures<br />
We examined endoscopist-reported variables of bowel preparation<br />
quality (Supplementary Table 2), time to maximum point of insertion,<br />
withdrawal time from maximum point of insertion, and segment of<br />
bowel reached. A pre-examination questionnaire assessed family<br />
history of CRC in first-degree relatives and a post-examination<br />
questionnaire assessed the level of pain experienced (none, mild,<br />
quite a lot, severe) during FS.<br />
Primary outcomes were distal adenomas and distal CRC incidence.<br />
Distal adenomas included adenomas detected at FS or any distal<br />
adenoma detected at follow-up colonoscopy (endoscopists were<br />
to leave polyps ≥ 10 mm for removal at colonoscopy). Distal CRCs,<br />
defined by the International Classification of Diseases 10th revision<br />
(ICD-10) and ICD for Oncology 2nd edition [22], included sites C18.7,<br />
C19 and C20 (rectum and sigmoid colon) and morphologies for<br />
invasive adenocarcinomas and carcinomas not otherwise specified<br />
for cancers diagnosed on clinical grounds only. The earliest distal<br />
CRC diagnosed per patient was included and follow- up time was not<br />
censored at diagnosis of proximal or unspecified site CRC.<br />
Statistical analysis<br />
Univariable and multivariable logistic regression was used to estimate<br />
odds ratios (OR) and 95% confidence intervals (CIs) for associations<br />
with distal adenoma detection. For distal CRC incidence, Cox models<br />
were used to estimate hazard ratios (HR) and 95% CIs. Time-at-risk<br />
started from baseline FS examination and was censored at emigration,<br />
death or the end of 2014. Non-proportionality was assessed using the<br />
Schoenfeld test; no violations were identified.<br />
Initial univariable analyses included everyone with complete data<br />
on each variable, referred to as “full dataset” analyses. Multivariable<br />
analyses required data for all variables in the model, referred to as<br />
“complete-case” analyses; see Tables 1 and 2 for details. Insertion and<br />
withdrawal times were missing in ~ 40% of participants as this was not<br />
recorded until partway through the trial. Further sensitivity analyses<br />
were conducted excluding participants with multiple FS examinations.<br />
Multivariable models were constructed based on a-priori plans using<br />
previous research [14, 23] and included: age, sex, family history of<br />
CRC, bowel preparation quality, insertion time, withdrawal time,<br />
segment of bowel reached and patient-reported pain. The multivariable<br />
model for distal adenoma detection also included centre while that for<br />
distal CRC incidence included endoscopist ADR group and centre was<br />
omitted due to collinearity with ADR group. Kaplan–Meier estimates<br />
show time to distal CRC diagnosis.<br />
Negative examinations were those with no findings in the colorectum<br />
(no lesions detected, no biopsies performed). Among those with<br />
negative examinations, we examined variation in KPIs, the associations<br />
between insertion time and pain and segment reached, and the<br />
Fig. 1 Study Profile. † 784 patients whose FS screening was performed by an endoscopist other than the main endoscopist at that centre, 536<br />
patients screened at one pilot centre that had far fewer participants than the other centres and where there were two main endoscopists rather<br />
than one, 367 patients who were screened within the first two months at one centre where the pathologist was found to be over-diagnosing<br />
adenomas, 93 participants with CRC diagnosed at baseline and 4,361 participants whose exam was classed as incomplete by the endoscopist. ‡ Four<br />
patients had incident CRC diagnosed at both sub-sites. § Three patients had CRC as the underlying cause of death but the sub-site specific cause<br />
could not be determined as CRC was diagnosed at both sub-sites<br />
associations between bowel preparation quality and pain and reaching<br />
the splenic flexure (SF). To examine if KPIs were associated with<br />
of 15%, 12% and 9%, respectively [15]; these groups Outcome permission ascertainment to obtain and process patient data (PIAG 4–07(j)/2002). All<br />
complexity of findings at FS, we investigated associations with the<br />
were used in this analysis. The order in which participants<br />
were screened revealed a learning effect for the and date of emigrations and deaths were collected from<br />
Information methods were on carried date, out site according and morphology to the relevant of guidelines. cancers<br />
outcome of detection of multiple adenomas and/or any advanced<br />
adenoma (defined as adenomas 10 mm, with high-grade dysplasia,<br />
endoscopists’ ADR [15]; thus, we created a variable that National Results cancer registries, the National Health Service<br />
or with villous/tubulovillous histology).<br />
grouped participants according to the order of examination<br />
occurrence: the first 500 participants examined NHS The Digital median and age the at FS Office was 60 for years, National 53% of Statistics. participants were males<br />
(NHS) Central Register, National Services Scotland,<br />
by Analyses each endoscopist were performed and using those STATA/IC examined V.13.1 (StataCorp later. LP, 2013;<br />
and 11% had ≥ 1 first degree relative with CRC (Table 1). Bowel<br />
Stata Statistical Software: Release 13; Texas, USA). Two-sided p-values<br />
< 0.05 were considered statistically significant. Ethical approval was<br />
preparation quality was excellent for 43%. Median insertion and<br />
obtained from local research ethics review committees for each centre withdrawal times were 2.4 (IQR 1.7–3.4) and 1.9 (IQR 1.2–3.4) minutes,<br />
(Multicentre Research Ethics Committee reference: 03/01/22). Trial respectively. Most examinations reached the descending colon or<br />
registration: ISRCTN28352761. All individuals who underwent FS further (78%) and 29% of participants reported feeling no pain during<br />
provided written informed consent prior to examination. The Patient<br />
Information Advisory Group (now Confidentiality Advisory Group) granted<br />
the examination (Table 1).<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
8 9
FEATURE<br />
Power et al. BMC <strong>Gastroenterology</strong> (<strong>2024</strong>) 24:44<br />
FEATURE<br />
Page 5 of 13<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
Variables were examined by centre, synonymous with endoscopist,<br />
among the 70% of participants with negative examinations<br />
(Supplementary Table 3). ‘Excellent’ bowel preparation quality varied<br />
between 9.6% (centre 9) and 68.2% (centre 4). Median insertion time<br />
varied from 1.45 (IQR 1.03–2.07; centre 4) to 3.88 (IQR, 2.92–5.50;<br />
centre 10) minutes and median withdrawal time varied from 0.88<br />
(IQR 0.65–1.27; centre 4) to 2.38 (IQR 1.90–3.06; centre 5) minutes.<br />
Examinations reaching the descending colon varied between 27.7%<br />
(centre 13) and 84.1% (centre 8) and participants reporting severe<br />
pain varied between 0.2% (centre 4) and 4.1% (centre 8). Despite<br />
these differences between centres, there were no clear associations<br />
between these factors and endoscopist ADR when examining by<br />
ascending order of ADR (Supplementary Table 3).<br />
Among negative examinations, the proportion of participants reporting<br />
quite a lot/severe pain tended to decrease with further segment reached<br />
(p-trends < 0.001). Females were more likely to report quite a lot/severe<br />
pain than males (15.5% vs. 8.0%, respectively, among exams reaching a<br />
maximum of the SF) and to have a longer time to maximum insertion for<br />
each section of the bowel reached (SF: median 2.37 min (IQR 1.75–3.45)<br />
vs. 2.14 min (IQR 1.58–2.90)) (Supplementary Table 4).<br />
In complete-case analyses, 3,349 (14.4%) negative examinations<br />
reached at least the SF. Females were less likely to have an<br />
examination reaching the SF (10.9%) than males (18.2%) (multivariable:<br />
OR 0.57, 95%CI 0.53– 0.62). Among those with negative exams, the<br />
odds of reaching the SF were 75% lower with ‘poor’ bowel preparation<br />
compared to ‘excellent’ (multivariable: OR 0.25 95%CI 0.13–0.50)<br />
and 47% lower with the reporting of severe pain compared to no pain<br />
(multivariable: OR 0.53 95%CI 0.38–0.73) (Supplementary Table 5).<br />
Distal adenoma detection<br />
There were 4,104 (12.0%) participants with ≥ 1 distal adenoma<br />
detected (Table 1). In all models, there were increased odds of distal<br />
adenoma detection with increasing age (multivariable: OR 1.03, 95%CI<br />
1.01–1.04) (Table 1), with a family history of CRC, compared to without<br />
(multivariable: OR 1.40, 95%CI 1.21–1.62), and decreased odds in<br />
females compared to males (multivariable: OR 0.62, 95%CI 0.56–0.69).<br />
Although there was no association in the full dataset, in complete-case<br />
models there were increased odds of distal adenoma detection for those<br />
with ‘poor’ bowel preparation compared to ‘excellent’ (multivariable:<br />
OR 2.88, 95%CI 1.25–6.60; Table 1), and lower odds for those with<br />
‘good’ (multivariable: OR 0.84, 95%CI 0.74–0.95) or ‘adequate’ bowel<br />
preparation (multivariable: OR 0.56, 95%CI 0.49–0.65).<br />
In all models, increasing insertion and withdrawal times were<br />
associated with distal adenoma detection (multivariable: OR ≥ 4.00<br />
vs. < 2.00 min: 1.96, 95%CI 1.68–2.29; 32.79, 95%CI 28.22–38.11,<br />
respectively). In comparison to reaching the sigmoid/descending<br />
junction, reaching more proximally was associated with higher odds<br />
of distal adenoma detection in univariable models (full dataset,<br />
descending colon: OR 1.43, 95%CI 1.31–1.56; SF: OR 1.66, 95%CI<br />
1.47–1.88); however, this attenuated in multivariable models.<br />
In complete-case univariable models, there were lower odds of distal<br />
adenoma detection with increasing pain (severe compared to none: OR<br />
0.69, 95%CI 0.51–0.95; Table 1) but this was not evident in the other<br />
models. In the full dataset, individuals whose FS screening occurred<br />
after their endoscopist’s first 500 examinations had increased odds of<br />
distal adenoma detection compared to those whose took place earlier<br />
(OR 1.32, 95%CI 1.20–1.45); multivariable models were not possible<br />
due to missing data.<br />
Advanced and/or multiple adenomas<br />
There were 919 (4.8%) participants with multiple and/ or advanced<br />
distal adenomas in the complete-case dataset (Supplementary Table<br />
6). Age, sex, family history, bowel preparation quality, insertion and<br />
withdrawal time, segment reached, patient pain and the order of FS<br />
occurrence were similarly associated with the detection of advanced<br />
and/or multiple adenomas as of any distal adenoma.<br />
Distal CRC incidence<br />
During a median follow-up of 17 years, 168 (0.5%) distal CRCs were<br />
diagnosed (Table 2). In the full dataset, females had a lower risk of<br />
distal CRC than males (HR 0.62, 95%CI 0.45–0.85) and those with a<br />
family history of CRC had a higher risk than those without (HR 1.65,<br />
95%CI 1.09–2.50) (Table 2, Supplementary Fig. 1A-B); these effects<br />
attenuated in complete-case models.<br />
Age, bowel preparation quality, segment of bowel reached, patientreported<br />
pain, and order of examination occurrence were not<br />
associated with distal CRC incidence (Table 2, Supplementary Fig. 1C–<br />
F). Although overall the associations for insertion and withdrawal times<br />
were not statistically significant, those in the top category of ≥ 4.00 min<br />
(versus < 2.00 min) had an increased risk of distal CRC (multivariable:<br />
HR 1.81, 95%CI 1.00–3.27; HR 1.93, 95%CI 1.14–3.24, respectively)<br />
(Table 2, Supplementary Fig. 1G-H).<br />
Compared to those examined by high-detectors, individuals examined<br />
by low-detectors had an increased risk of distal CRC (multivariable:<br />
HR 4.71, 95%CI 2.65–8.38), as did those examined by intermediatedetectors<br />
in complete- case models only (multivariable: HR 2.16,<br />
95%CI 1.22–3.81) (Table 2, Supplementary Fig. 1I).<br />
Excluding participants with multiple FS examinations (n = 1,810) did not<br />
materially alter the results for distal adenoma detection or long-term<br />
colorectal cancer incidence in any of the models.<br />
Discussion<br />
We investigated factors that could improve the quality of FS<br />
examinations, increase adenoma detection, and reduce CRC<br />
incidence. We found that multiple variables were associated with<br />
adenoma detection, including patient age, sex, family history of CRC,<br />
bowel preparation quality, insertion time and withdrawal time. For longterm<br />
outcomes, patients who were examined by endoscopists with<br />
higher ADRs had a lower risk of distal CRC incidence.<br />
Individuals with a family history of CRC or its precursor lesions are<br />
at increased risk of CRC compared to those without [24]; similarly,<br />
we found a positive association between family history of CRC and<br />
distal adenoma detection at FS screening [25]. Participants provided<br />
family history information on the pre-screening questionnaire, which<br />
the endoscopist may have accessed, potentially motivating them to<br />
conduct a more thorough FS examination.<br />
Table 1 Detection of any distal adenoma by patient factors and endoscopist variables<br />
All eligible participants: Full dataset analysis (n = 34 139) Participants with complete data on all variables: Complete-case analysis (n = 19 333) a<br />
p-value Multivariable OR p-value<br />
(95%CI) c<br />
Univariable OR<br />
(95%CI)<br />
p-value n (%) b Participants<br />
with ≥ 1 adenoma<br />
detected at<br />
baseline n (%)<br />
Univariable OR<br />
(95%CI)<br />
n (%) b Participants<br />
with ≥ 1 adenoma<br />
detected at<br />
baseline n (%)<br />
Total 34 139 (100) 4 104 (12.0) 19 333 (100) 2 415 (12.5)<br />
Age (IQR), years 60.3 (57.9–62.8) 4 104 (12.0) 1.03 (1.02–1.04) < 0.001 60.5 (58.0–62.9) 2 415 (12.5) 1.03 (1.01–1.05) < 0.001 1.03 (1.01–1.04) 0.002<br />
Sex 34 139 (100) 4 104 (12.0) < 0.001 < 0.001 < 0.001<br />
Male 18 127 (53.1) 2 817 (15.5) 1 10 315 (53.4) 1 665 (16.1) 1 1<br />
Female 16 012 (46.9) 1 287 (8.0) 0.48 (0.44–0.51) 9 018 (46.6) 750 (8.3) 0.47 (0.43–0.52) 0.62 (0.56–0.69)<br />
32 356 (94.8) 3 926 (12.1) < 0.001 < 0.001 < 0.001<br />
Family history of<br />
CRC<br />
No 28 663 (88.6) 3 381 (11.8) 1 17 123 (88.6) 2 077 (12.1) 1 1<br />
Yes 3 693 (11.4) 545 (14.8) 1.29 (1.17–1.43) 2 210 (11.4) 338 (15.3) 1.31 (1.15–1.48) 1.40 (1.21–1.62)<br />
Centre 34 139 (100) 4 104 (12.0) < 0.001 < 0.001 < 0.001<br />
1 2 413 (7.1) 207 (8.6) 1 1 607 (8.3) 139 (8.6) 1 1<br />
2 d 3 438 (10.1) 302 (8.8) 1.03 (0.85–1.23) - - - -<br />
3 2 674 (7.8) 249 (9.3) 1.09 (0.90–1.33) 1 452 (7.5) 135 (9.3) 1.08 (0.84–1.39) 0.91 (0.67–1.23)<br />
4 2 131 (6.2) 209 (9.8) 1.16 (0.95–1.42) 1 452 (7.5) 146 (10.1) 1.18 (0.93–1.51) 1.55 (1.14–2.09)<br />
5 2 466 (7.2) 271 (11.0) 1.32 (1.09–1.59) 1 844 (9.5) 189 (10.2) 1.21 (0.96–1.52) 0.41 (0.31–0.54)<br />
6 2 733 (8.0) 306 (11.2) 1.34 (1.12–1.62) 1 579 (8.2) 163 (10.3) 1.22 (0.96–1.54) 1.23 (0.92–1.65)<br />
7 2 516 (7.4) 282 (11.2) 1.35 (1.11–1.62) 1 741 (9.0) 205 (11.8) 1.41 (1.12–1.77) 1.19 (0.89–1.58)<br />
8 2 839 (8.3) 362 (12.8) 1.56 (1.30–1.86) 1 486 (7.7) 190 (12.8) 1.55 (1.23–1.95) 0.77 (0.58–1.03)<br />
9 2 493 (7.3) 349 (14.0) 1.73 (1.45–2.08) 1 617 (8.4) 236 (14.6) 1.80 (1.45–2.25) 1.39 (1.06–1.83)<br />
10 2 532 (7.4) 370 (14.6) 1.82 (1.52–2.18) 1 578 (8.2) 215 (13.6) 1.67 (1.33–2.09) 0.57 (0.43–0.76)<br />
11 2 324 (6.8) 347 (14.9) 1.87 (1.56–2.25) 1 202 (6.2) 185 (15.4) 1.92 (1.52–2.43) 0.65 (0.49–0.86)<br />
12 2 799 (8.2) 421 (15.0) 1.89 (1.58–2.25) 2 214 (11.5) 348 (15.7) 1.97 (1.60–2.43) 1.11 (0.86–1.44)<br />
13 2 781 (8.1) 429 (15.4) 1.94 (1.63–2.32) 1 561 (8.1) 264 (16.9) 2.15 (1.73–2.67) 0.71 (0.54–0.92)<br />
33 609 (98.4) 3 925 (11.7) 0.05 0.001 < 0.001<br />
Bowel preparation<br />
quality<br />
Excellent 14 573 (43.4) 1 690 (11.6) 1 7 819 (40.4) 924 (11.8) 1 1<br />
Good 11 692 (34.8) 1 431 (12.2) 1.06 (0.99–1.15) 6 922 (35.8) 918 (13.3) 1.14 (1.03–1.26) 0.84 (0.74–0.95)<br />
Adequate 7 060 (21.0) 769 (10.9) 0.93 (0.85–1.02) 4 553 (23.6) 561 (12.3) 1.05 (0.94–1.17) 0.56 (0.49–0.65)<br />
Poor 284 (0.8) 35 (12.3) 1.07 (0.75–1.53) 39 (0.2) 12 (30.8) 3.32 (1.67–6.57) 2.88 (1.25–6.60)<br />
Insertion time 20 371 (59.7) 2 630 (12.9) < 0.001 < 0.001 < 0.001<br />
< 2.00 mins 7 357 (36.1) 795 (10.8) 1 7 014 (36.3) 727 (10.4) 1 1<br />
2.00–2.59 mins 6 198 (30.4) 759 (12.2) 1.15 (1.04–1.28) 5 894 (30.5) 702 (11.9) 1.17 (1.05–1.31) 1.18 (1.04–1.35)<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
10 11
FEATURE<br />
Power et al. BMC <strong>Gastroenterology</strong> (<strong>2024</strong>) 24:44<br />
Page 6 of 13<br />
Power et al. BMC <strong>Gastroenterology</strong> (<strong>2024</strong>) 24:44<br />
FEATURE<br />
Page 7 of 13<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
Table 1 (continued)<br />
All eligible participants: Full dataset analysis (n = 34 139) Participants with complete data on all variables: Complete-case analysis (n = 19 333) a<br />
n (%) b Participants<br />
with ≥ 1 adenoma<br />
detected at<br />
baseline n (%)<br />
Univariable OR<br />
(95%CI)<br />
p-value n (%) b Participants<br />
with ≥ 1 adenoma<br />
detected at<br />
baseline n (%)<br />
Univariable OR<br />
(95%CI)<br />
p-value Multivariable OR p-value<br />
(95%CI) c<br />
3.00–3.59 mins 3 412 (16.7) 466 (13.7) 1.31 (1.16–1.48) 3 221 (16.7) 424 (13.2) 1.31 (1.15–1.49) 1.37 (1.18–1.61)<br />
≥ 4.00 mins 3 404 (16.7) 610 (17.9) 1.80 (1.61–2.02) 3 204 (16.6) 562 (17.5) 1.84 (1.63–2.07) 1.96 (1.68–2.29)<br />
Withdrawal time 20 326 (59.5) 2 621 (12.9) < 0.001 < 0.001 < 0.001<br />
< 2.00 mins 10 625 (52.3) 295 (2.8) 1 10 204 (52.8) 262 (2.6) 1 1<br />
2.00–2.59 mins 3 672 (18.1) 295 (8.0) 3.06 (2.59–3.61) 3 479 (18.0) 266 (7.6) 3.14 (2.64–3.74) 3.85 (3.21–4.62)<br />
3.00–3.59 mins 1 837 (9.0) 311 (16.9) 7.14 (6.03–8.44) 1 740 (9.0) 289 (16.6) 7.56 (6.34–9.01) 9.47 (7.88–11.38)<br />
≥ 4.00 mins 4 192 (20.6) 1 720 (41.0) 24.36 (21.37–27.78) 3 910 (20.2) 1 598 (40.9) 26.23 (22.84–30.12) 32.79 (28.22–38.11)<br />
Segment reached 34 075 (99.8) 4 098 (12.0) < 0.001 < 0.001 0.79<br />
RM/RS/SC 128 (0.4) 16 (12.5) 1.43 (0.84–2.44) 53 (0.3) 8 (15.1) 1.63 (0.76–3.48) 1.12 (0.46–2.69)<br />
SD 7 510 (22.0) 680 (9.1) 1 4 447 (23.0) 437 (9.8) 1 1<br />
DC 21 327 (62.6) 2 660 (12.5) 1.43 (1.31–1.56) 11 840 (61.2) 1 519 (12.8) 1.35 (1.21–1.51) 1.00 (0.87–1.14)<br />
SF 3 462 (10.2) 491 (14.2) 1.66 (1.47–1.88) 2 062 (10.7) 310 (15.0) 1.62 (1.39–1.90) 1.12 (0.92–1.38)<br />
TC/HF/AC/CM/TI 1 648 (4.8) 251 (15.2) 1.80 (1.54–2.11) 931 (4.8) 141 (15.1) 1.64 (1.33–2.01) 1.01 (0.79–1.28)<br />
Patient pain 33323 (97.6) 3 989 (12.0) 0.66 0.003 0.48<br />
None 9 563 (28.7) 1 164 (12.2) 1 4 937 (25.5) 685 (13.9) 1 1<br />
Mild 17 859 (53.6) 2 139 (12.0) 0.98 (0.91–1.06) 10 561 (54.6) 1 292 (12.2) 0.87 (0.78–0.96) 0.92 (0.82–1.03)<br />
Quite a lot 5 224 (15.7) 613 (11.7) 0.96 (0.86–1.06) 3 378 (17.5) 392 (11.6) 0.81 (0.71–0.93) 0.92 (0.78–1.07)<br />
Severe 677 (2.0) 73 (10.8) 0.87 (0.68–1.12) 457 (2.4) 46 (10.1) 0.69 (0.51–0.95) 0.86 (0.60–1.25)<br />
FS occurrence e 34 139 (100) 4 104 (12.0) < 0.001 - -<br />
First group 500 5 410 (15.8) 526 (9.7) 1 - - - -<br />
Later groups 500 28 729 (84.2) 3 578 (12.5) 1.32 (1.20–1.45) - - - -<br />
Abbreviations: AC ascending colon, CI confidence interval, CM caecum, DC descending colon, FS flexible sigmoidoscopy, HF hepatic flexure, Mins minutes, OR odds ratio, RM rectum, RS recto sigmoid, SC sigmoid colon, SD<br />
sigmoid descending, SF splenic flexure, TC transverse colon, TI terminal ileum<br />
P-values were calculated with the likelihood ratio test<br />
a 1 783 missing values on family history of CRC; 530 missing values on bowel preparation quality; 13 768 missing values on insertion time; 13 813 missing values on withdrawal time; 64 missing values on segment<br />
reached; 816 missing values on patient-reported pain (these values are not mutually exclusive)<br />
b All n and percentage except the entry for age, which is median and interquartile range<br />
c Multivariable model includes age, sex, family history of CRC, centre, bowel preparation quality, insertion time, withdrawal time, segment reached and patient-reported pain<br />
d<br />
Centre 2 was omitted from the complete-case analyses due to a lack of recorded information for insertion or withdrawal times, as this information was not required until partway through the trial at which time centre 2<br />
had already completed recruitment<br />
e Order of occurrence of FS examination was omitted from the complete-case analyses due to a lack of recorded information for insertion and withdrawal times for the category ‘first group 500’; this information was not<br />
required until partway through the trial at which time each endoscopist had already completed 500 examinations<br />
Table 2 Long-term distal colorectal cancer incidence by patient factors and endoscopist variables<br />
All eligible participants: Full dataset analysis (n = 34 139) Participants with complete data on all variables: Complete-case analysis (n = 19 294) a,b<br />
n (%) c Number of<br />
distal CRCs, n<br />
Incidence rate<br />
per 100,000<br />
person-years<br />
(95% CI)<br />
Univariable HR<br />
(95%CI)<br />
p-value n (%) c Number of<br />
distal CRCs, n<br />
Incidence rate<br />
per 100,000<br />
person-years<br />
(95% CI)<br />
Univariable HR<br />
(95%CI)<br />
p-value Multivariable p-value<br />
HR (95%CI) d<br />
Total 34 139 (100) 168 31.4 (27.0–36.5) 19 294 (100) 91 30.8 (25.1–37.9)<br />
Age (IQR), years 60.3 (57.9–62.8) 168 - 1.03 (0.98–1.09) 0.23 60.5 (58.0–62.9) 91 - 1.00 (0.93–1.08) 0.98 1.00 (0.93–1.08) 0.96<br />
Sex 34 139 (100) 168 0.003 0.06 0.12<br />
Male 18 127 (53.1) 106 38.1 (31.5–46.1) 1 10 294 (53.4) 56 36.2 (27.9–47.1) 1 1<br />
Female 16 012 (46.9) 62 24.1 (18.8–31.0) 0.62 (0.45–0.85) 9 000 (46.7) 35 24.9 (17.9–34.7) 0.67 (0.44–1.03) 0.71 (0.45–1.09)<br />
Family history<br />
of CRC<br />
32 356 (94.8) 156 0.026 0.23 0.24<br />
No 28 663 (88.6) 129 28.8 (24.2–34.2) 1 17 087 (88.6) 77 29.4 (23.5–36.8) 1 1<br />
Yes 3 693 (11.4) 27 47.1 (32.3–68.7) 1.65 (1.09–2.50) 2 207 (11.4) 14 42.0 (24.9–70.9) 1.44 (0.81–2.54) 1.43 (0.81–2.52)<br />
Bowel preparation<br />
quality<br />
33 609 (98.4) 164 0.19 0.45 0.16<br />
Excellent 14 573 (43.4) 62 26.8 (20.9–34.4) 1 7 819 (40.5) 33 27.4 (19.5–38.5) 1 1<br />
Good 11 692 (34.8) 56 30.7 (23.6–39.9) 1.15 (0.80–1.65) 6 922 (35.9) 32 30.3 (21.4–42.9) 1.11 (0.69–1.81) 1.24 (0.76–2.03)<br />
Adequate 7 060 (21.0) 44 40.5 (30.1–54.4) 1.53 (1.04–2.25) 4 553 (23.6) 26 37.8 (25.7–55.5) 1.40 (0.83–2.33) 1.72 (0.99–2.97)<br />
Poor b 284 (0.8) 2 44.8 (11.2–178.9) 1.65 (0.40–6.74) - - - - -<br />
Insertion time 20 371 (59.7) 93 0.53 0.42 0.20<br />
< 2.00 mins 7 357 (36.1) 31 27.6 (19.4–39.2) 1 7 006 (36.3) 30 28.0 (19.6–40.1) 1 1<br />
2.00–2.59 mins 6 198 (30.4) 27 28.5 (19.5–41.6) 1.03 (0.62–1.73) 5 885 (30.5) 27 30.0 (20.6–43.7) 1.07 (0.63–1.79) 1.09 (0.64–1.84)<br />
3.00–3.59 mins 3 412 (16.7) 14 26.7 (15.8–45.1) 0.96 (0.51–1.81) 3 216 (16.7) 13 26.3 (15.3–45.3) 0.93 (0.49–1.79) 0.95 (0.49–1.86)<br />
≥ 4.00 mins 3 404 (16.7) 21 40.6 (26.5–62.3) 1.47 (0.84–2.55) 3 187 (16.5) 21 43.3 (28.2–66.4) 1.54 (0.88–2.69) 1.81 (1.00–3.27)<br />
Withdrawal<br />
time<br />
20 326 (59.5) 93 0.24 0.27 0.09<br />
< 2.00 mins 10 625 (52.3) 42 25.8 (19.1–34.9) 1 10 190 (52.8) 42 26.9 (19.9–36.4) 1 1<br />
2.00–2.59 mins 3 672 (18.1) 15 26.5 (16.0–44.0) 1.02 (0.56–1.83) 3 469 (18.0) 15 28.1 (16.9–46.6) 1.03 (0.57–1.86) 1.00 (0.55–1.82)<br />
3.00–3.59 mins 1 837 (9.0) 9 32.0 (16.7–61.5) 1.23 (0.60–2.52) 1 738 (9.0) 8 30.1 (15.1–60.2) 1.11 (0.52–2.36) 1.19 (0.55–2.57)<br />
≥ 4.00 mins 4 192 (20.6) 27 42.7 (29.3–62.3) 1.64 (1.01–2.67) 3 897 (20.2) 26 44.2 (30.1–64.9) 1.63 (1.00–2.66) 1.93 (1.14–3.24)<br />
Segment<br />
reached<br />
34 075 (99.8) 167 0.50 0.42 0.45<br />
RM/RS/SC/SD 7 638 (22.4) 36 30.0 (21.7–41.7) 1 4 484 (23.2) 18 26.2 (16.5–41.6) 1 1<br />
DC 21 327 (62.6) 111 33.2 (27.6–40.0) 1.11 (0.76–1.61) 11 822 (61.3) 62 34.2 (26.7–43.9) 1.30 (0.77–2.20) 0.99 (0.57–1.71)<br />
SF/TC/HF/AC/<br />
CM/TI<br />
5 110 (15.0) 20 25.1 (16.2–39.0) 0.84 (0.49–1.46) 2 988 (15.5) 11 24.3 (13.5–43.9) 0.93 (0.44–1.98) 0.67 (0.31–1.44)<br />
Patient pain 33 323 (97.6) 163 0.25 0.49 0.81<br />
None 9 563 (28.7) 55 36.7 (28.2–47.9) 1 4 929 (25.5) 28 37.4 (25.8–54.1) 1 1<br />
Mild 17 859 (53.6) 85 30.4 (24.6–37.6) 0.83 (0.59–1.16) 10 541 (54.6) 47 29.1 (21.9–38.8) 0.78 (0.49–1.24) 0.87 (0.54–1.40)<br />
12 13
f<br />
FEATURE<br />
Power et al. BMC <strong>Gastroenterology</strong> (<strong>2024</strong>) 24:44<br />
Page 8 of 13<br />
FEATURE<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
Table 2 (continued)<br />
All eligible participants: Full dataset analysis (n = 34 139) Participants with complete data on all variables: Complete-case analysis (n = 19 294) a,b<br />
p-value Multivariable p-value<br />
HR (95%CI) d<br />
Univariable HR<br />
(95%CI)<br />
Incidence rate<br />
per 100,000<br />
person-years<br />
(95% CI)<br />
p-value n (%) c Number of<br />
distal CRCs, n<br />
Univariable HR<br />
(95%CI)<br />
Incidence rate<br />
per 100,000<br />
person-years<br />
(95% CI)<br />
n (%) c Number of<br />
distal CRCs, n<br />
Quite a lot/ 5 901 (17.7) 23 24.8 (16.5–37.3) 0.68 (0.42–1.10) 3 824 (19.8) 16 27.2 (16.7–44.4) 0.73 (0.39–1.34) 0.83 (0.44–1.58)<br />
severe e<br />
FS occurrence f 34 139 (100) 168 0.18 - - - -<br />
First group 500 5 410 (15.8) 35 39.8 (28.5–55.4) 1 - - - - - -<br />
28 729 (84.2) 133 29.8 (25.1–35.3) 0.77 (0.53–1.12) - - - - - -<br />
Later groups<br />
500<br />
34 139 (100) 168 0.001 < 0.001 < 0.001<br />
Endoscopist’s<br />
ADR ranking<br />
group<br />
High 12 929 (37.9) 44 21.8 (16.2–29.3) 1 8 161 (42.3) 22 17.5 (11.5–26.6) 1 1<br />
Intermediate 10 554 (30.9) 51 30.9 (23.5–40.7) 1.42 (0.95–2.13) 6 626 (34.3) 32 31.5 (22.3–44.5) 1.81 (1.05–3.12) 2.16 (1.22–3.81)<br />
Low 10 656 (31.2) 73 43.5 (34.6–54.7) 1.99 (1.37–2.90) 4 507 (23.4) 37 54.8 (39.7–75.6) 3.24 (1.91–5.49) 4.71 (2.65–8.38)<br />
Abbreviations: AC ascending colon, ADR adenoma detection rate, CI confidence interval, CM caecum, CRC colorectal cancer, DC descending colon, FS flexible sigmoidoscopy, HF hepatic flexure, HR hazard ratio, Mins<br />
minutes, RM rectum, RS recto sigmoid, SC sigmoid colon, SD sigmoid descending, SF splenic flexure, TC transverse colon, TI terminal ileum<br />
P-values were calculated with the likelihood ratio test<br />
a<br />
1 783 missing values on family history; 530 missing values on bowel preparation quality; 13 768 missing values on insertion time; 13 813 missing values on withdrawal time; 64 missing values on segment reached; 816<br />
missing values on patient-reported pain (these values are not mutually exclusive)<br />
b Participants with the ‘poor’ category of bowel preparation quality (n = 39) were excluded from the complete-case analyses due to a lack of cases<br />
c<br />
All n and percentage except the entry for age, which is median and interquartile range<br />
d<br />
Multivariable model includes age, sex, family history of CRC, bowel preparation quality, insertion time, withdrawal time, segment reached, patient-reported pain and endoscopist’s ADR ranking group<br />
e<br />
Participants with the ‘severe’ category of patient-reported pain were combined with the category ‘quite a lot’ due to a lack of cases<br />
Order of occurrence of FS examination was omitted from the complete-case analyses due to a lack of recorded information for the variables of insertion and withdrawal time for the category ‘first group 500’; this<br />
information was not required until partway through the trial at which time each endoscopist had already completed 500 examinations<br />
Bowel preparation plays a crucial role in the quality and completeness reached, insertion time and withdrawal time. Quality of bowel<br />
of endoscopic examinations, with higher levels of cleanliness<br />
preparation has been associated with longer insertion times [30, 31].<br />
associated with optimum views of the colon [26] and improved ADRs Advancing a sigmoidoscope through a bowel with poorer preparation<br />
[23]. Compared to having ‘excellent’ bowel preparation, we found lower requires more cleaning to obtain good views of the mucosa, potentially<br />
odds of adenoma detection among those having ‘good’ or ‘adequate’ increasing insertion times. However, in our multivariable model<br />
and increased odds among those having ‘poor’. Among participants including both insertion time and bowel preparation quality, the<br />
with poor bowel preparation at first FS, those who had adenomas association between insertion time and adenoma detection remained.<br />
detected that triggered referral to colonoscopy would not have had a<br />
repeat FS to improve the bowel preparation quality; however, those Higher quality withdrawal techniques are associated with fewer missed<br />
without high-risk adenomas detected would have undergone a repeat adenomas. Colonoscopists with lower miss rates for adenomas had<br />
FS, likely improving the bowel preparation quality. This could contribute longer examination times compared to those with higher miss rates<br />
to poor bowel preparation being positively associated with adenoma [19]. We found that longer withdrawal times were associated with<br />
detection. We were unable to examine poor bowel preparation quality increased adenoma detection, which is unsurprising due to the time<br />
and distal CRC incidence due to a lack of cases, attributed to the fact taken to remove lesions < 10 mm during FS. Although larger lesions<br />
that we only included complete examinations.<br />
would not have been removed during FS, they would likely increase<br />
the examination time. For distal CRC incidence, only the longest<br />
In contrast to previous findings, which either reported no correlation withdrawal time category was associated with increased risk; this<br />
between adenoma detection and longer insertion time [27] or<br />
can likely be attributed to patients with long withdrawal times having<br />
decreased adenoma detection with longer insertion times [28, 29], more advanced pathology and inherently being at higher risk rather<br />
we found that longer insertion time was associated with greater than reflecting the quality of the endoscopist’s withdrawal. There is no<br />
adenoma detection. Within the UKFSST, endoscopists were to<br />
minimum recommended withdrawal time for FS, unlike for colonoscopy<br />
remove polyps ≤ 5 mm during insertion to avoid difficulties relocating [20]. A previous study suggested a FS withdrawal time of at least 3.25<br />
them on withdrawal, remove polyps 6-9 mm during withdrawal, and min from the SF and, to maximise ADRs, specified an aim of 3.5–4.0<br />
leave polyps ≥ 10 mm for removal at colonoscopy. Therefore, longer minutes [14]; although this has not been validated, our data supports<br />
insertion times in this study could be associated with the presence this recommendation.<br />
of numerous polyps ≤ 5 mm requiring resection and/or very large<br />
adenomas needing endoscopic assessment/ photo-documentation. FS with a 60 cm maximum scope insertion distance can reach the<br />
We also found increased odds of detecting multiple and/or advanced SF and sometimes beyond [32]. In our study, the majority (78%) of<br />
adenomas in those with longer insertion times. In our study there examinations were judged by the endoscopist to have reached at least<br />
was no fixed endpoint for FS examinations and further reach of the the descending colon with 15% reaching at least the SF. It is important<br />
sigmoidoscope during an examination would naturally lead to longer that the sigmoidoscope reaches as high as comfortably possible<br />
insertion and withdrawal times and a higher chance of adenoma to maximise the mucosa examined, increasing the effectiveness of<br />
detection. However, in multivariable models we adjusted for segment the examination [33, 34]. In our univariable analyses, the chance of<br />
Publishers Comment<br />
For over 30 years thanks to trade support, we have been able to provide those working within <strong>Gastroenterology</strong> Departments<br />
and Endoscopy Units with quarterly copies of <strong>Gastroenterology</strong> <strong>Today</strong> free of charge in the knowledge that those receiving<br />
our dedicated publication enjoy having something to pick up and read during their free time. As in the current climate, return on<br />
investment appears to be the buzz word amongst suppliers, we would appreciate you mentioning <strong>Gastroenterology</strong> <strong>Today</strong><br />
when enquiring about products advertised.<br />
In respect of this current issue we would like to thank the following companies for their advertising support as without their<br />
contribution towards our print and postal costs this issue would not have been published. Alpha Laboratories, Biohit, Steris,<br />
Infai, Micro Tech, Nordic, Tillots.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
14 15
FEATURE<br />
FEATURE<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
detecting an adenoma was greater when at least the descending colon<br />
was reached, although this effect attenuated in multivariable models.<br />
Previously, inadequate examinations (e.g., insertion of the scope <<br />
50 cm) were associated with female sex and advancing age, with the<br />
majority of incomplete examinations due to patient discomfort [34].<br />
In agreement with this, we found decreased odds of reaching the SF<br />
for females, those who reported more pain, and those with poorer<br />
bowel preparation quality among those with negative examinations<br />
[14]. However, we found no clear association between patient-reported<br />
pain and adenoma detection or distal CRC incidence. Identifying<br />
factors that could reduce levels of pain could result in more complete<br />
examinations, lessening the chances of negative experiences that<br />
could compromise attendance at future examinations.<br />
We found that adenomas were more likely to be detected at<br />
examinations conducted after an endoscopist’s first 500 examinations,<br />
suggesting a learning effect, consistent with previous analyses [15];<br />
although we cannot be certain that participants examined within an<br />
endoscopist’s first 500 examinations had adenomas missed at baseline.<br />
It has been reported that for each 1% increase in the ADR, there is an<br />
associated 3% decreased risk of post-colonoscopy CRC [13] and that<br />
greater long-term protection from CRC is observed when FS screening<br />
is conducted by endoscopists with higher ADRs [35]. We found an<br />
almost five-fold increase in distal CRC incidence for individuals screened<br />
by low-detectors compared to those screened by high-detectors;<br />
this suggests an ADR of 15%, observed among the high-detectors,<br />
should be considered as a minimal standard. Other factors could<br />
account for differences in ADR, including variations in equipment,<br />
screening protocols or endoscopists’ prior experience; these factors<br />
were controlled for in the study design/analysis, which lends more<br />
weight to the difference in ADRs reflecting real variability in endoscopist<br />
performance and consequent effects on CRC incidence [5].<br />
Although seven variables were associated with adenoma detection,<br />
only endoscopist ADR group was associated with distal CRC, in<br />
addition to insertion and withdrawal times in the top categories only.<br />
These differences potentially demonstrate the importance of certain<br />
factors in adenoma detection but not necessarily cancer prevention,<br />
but differences in findings could be due to a lack of power for the distal<br />
CRC analyses.<br />
Strengths of our study include the large, high-quality dataset with<br />
multiple KPI measures and long follow-up period. Participants were<br />
recruited throughout the UK, resulting in good generalisability of our<br />
findings. Complete endoscopic examinations are crucial as incomplete<br />
examinations are associated with higher numbers of interval cancers<br />
[36, 37]; we only included examinations classed by the endoscopist as<br />
complete. In addition, we only included examinations performed by the<br />
main endoscopist at each centre, which removed heterogeneity within<br />
centres introduced by multiple endoscopists. There were limitations,<br />
including missing data for insertion and withdrawal times, potential<br />
inaccuracy in classifying depth of insertion since imaging systems<br />
were not used and limited statistical power for distal CRC analyses.<br />
We were unable to exclude examination time used for polyp removal<br />
or endoscopic assessment/photo documentation of polyps, which<br />
may have contributed to the association between adenoma detection<br />
and longer insertion and withdrawal times. Additionally, since the trial<br />
screening was conducted there have been advances in the quality of<br />
endoscopic equipment and improvements in endoscopist training and<br />
monitoring; therefore, the number of adenomas detected today would<br />
likely be higher.<br />
In conclusion, there is a lack of published data on KPIs and long-term<br />
CRC outcomes. Examining the impact of KPIs on adenoma detection<br />
and distal CRC incidence, we identified several variables associated<br />
with patient outcomes. Examinations with good or adequate bowel<br />
preparation quality had lower odds of adenoma detection, and longer<br />
insertion and withdrawal times had increased odds of adenoma<br />
detection. Patients examined by endoscopists with high ADRs had the<br />
lowest risk of distal CRC. We suggest an ADR of 15% should be set as<br />
a minimal standard. The importance of the detection and removal of<br />
adenomas cannot be understated; early detection of abnormalities is<br />
key in providing long-term protection against CRC. It is vital that each<br />
endoscopic procedure is conducted to the highest standard, so all<br />
patients receive the optimum benefit that screening can offer.<br />
Parts of the reported results have been presented as a poster<br />
presentation [38].<br />
Abbreviations<br />
AC Ascending colon<br />
ADR Adenoma detection rate<br />
CI Confidence interval<br />
CM Caecum<br />
CRC Colorectal cancer<br />
DC Descending colon<br />
FS Flexible sigmoidoscopy<br />
HF Hepatic flexure<br />
HR Hazard ratio<br />
ICD-10 International Classification of Diseases 10th revision<br />
ISRCTN International Standard Randomised Controlled Trial Number<br />
IQR Interquartile range<br />
KPIs Key performance indicators<br />
Mins Minutes<br />
NHS National Health Service<br />
OR Odds ratio<br />
PIAG Patient Information Advisory Group<br />
RM Rectum<br />
RS Rectosigmoid<br />
SC Sigmoid colon<br />
SD Sigmoid descending<br />
SF Splenic flexure<br />
TC Transverse colon<br />
TI Terminal ileum<br />
UKFSST UK Flexible Sigmoidoscopy Screening Trial<br />
Supplementary Information<br />
The online version contains supplementary material available at<br />
https://doi.org/10.1186/s12876-024-03125-x.<br />
Additional file 1: Supplementary Table 1. Reasons for repeat flexible<br />
sigmoidoscopy. Supplementary Table 2. Protocol guidelines for the<br />
categorisation of bowel preparation quality. Supplementary Table 3.<br />
Endoscopist variables by endoscopist for negative examinations*.<br />
Supplementary Table 4. Patient-reported pain and insertion time<br />
by extent of examination in negative examinations*. Supplementary<br />
Table 5. Reaching the splenic flexure in negative examinations*<br />
by age, sex, bowel preparation quality, and pain. Supplementary<br />
Table 6. Detection of multiple and/or advanced distal adenomas by<br />
patient factors and endoscopist variables. Supplementary Figure 1.<br />
Cumulative distal colorectal cancer incidence in all eligible participants<br />
by patient factors and endoscopist variables (the full dataset).<br />
Acknowledgements<br />
CSPRG: Wendy Atkin, Mariano Perdices Kalfors, Paul Greliak, Iain<br />
Stenson, Salman Shahrezaei. Trial Steering Committee: M Parmar<br />
(Chair), R Valori, A Gray, J Patnick, A Mackie, L Berkman. We thank the<br />
general practitioners, hospital staff, and the men and women who took<br />
part in this study.<br />
Authors’ contributions<br />
SP, KW and AJC: conception and design; analysis and interpretation of<br />
the data; drafting of the article; All authors: critical revision of the article<br />
for important intellectual content and final approval of the article.<br />
Funding<br />
Currently, this trial is funded by the National Institute for Health<br />
Research (NIHR) Health Technology Assessment (HTA) ref: 16/65/01.<br />
The work of the Cancer Screening and Prevention Research Group<br />
(CSPRG) at Imperial College London is also supported by Cancer<br />
Research UK (C53889/A25004). SP is funded by a Cancer Research<br />
UK studentship award (A27007).<br />
Availability of data and materials<br />
The data used in this current study is not available as it uses individuallevel<br />
identifiable data, which is confidential. Requests regarding data<br />
should be directed to the corresponding author.<br />
Declarations<br />
Ethics approval and consent to participate<br />
Ethical approval was obtained from local research ethics review<br />
committees for each centre (Multicentre Research Ethics Committee<br />
reference: 03/01/22). Trial registration: ISRCTN28352761. All<br />
individuals who underwent FS provided written informed consent<br />
prior to examination. The Patient Information Advisory Group (now<br />
Confidentiality Advisory Group) granted permission to obtain and<br />
process patient data (PIAG 4–07(j)/2002). All the methods were carried<br />
out according to the relevant guidelines.<br />
Consent for publication<br />
Not applicable.<br />
Competing interests<br />
BPS has done consultancy work, been a speaker for, had loan<br />
equipment and a research grant from Olympus in the last 3 years; all<br />
other authors report no conflicts of interest.<br />
Author details<br />
1<br />
Cancer Screening and Prevention Research Group (CSPRG),<br />
Department of Surgery and Cancer, St Mary’s Hospital, Imperial<br />
College London, London W2 1NY, UK. 2 Department of Surgery<br />
and Cancer, Imperial College London, London, UK. 3 Department<br />
of <strong>Gastroenterology</strong>, St Mark’s Hospital and Academic Institute,<br />
London, UK.<br />
Received: 28 June 2023 Accepted: 2 January <strong>2024</strong><br />
Published online: 23 January <strong>2024</strong><br />
References<br />
1. Cancer Research UK. Bowel cancer statistics - bowel cancer<br />
incidence. Available from: www.cancerresearchuk.org/healthprofessional/cancer-statistics/statistics-by-cancer-type/bowelcancer#heading-Zero.<br />
Accessed Jan 2021.<br />
2. Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D,<br />
Andrews KS, et al. Screening and surveillance for the early<br />
detection of colorectal cancer and adenomatous polyps, 2008:<br />
a joint guideline from the American Cancer Society, the US<br />
Multi-Society Task Force on Colorectal Cancer, and the American<br />
College of Radiology. CA Cancer J Clin. 2008;58(3):130–60.<br />
3. Zauber A, Winawer S, O’Brien M, Landsdorp-Vogelaar I, van<br />
Ballegooijen M, Hankey B, et al. Colonoscopic polypectomy and<br />
long-term prevention of colorectal-cancer deaths. N Engl J Med.<br />
2012;336(8):687–96.<br />
4. Cunningham D, Atkin W, Lenz H-J, Lynch H, Minsky B, Nordlinger<br />
B, et al. Colorectal cancer. Lancet. 2010;375:1030–47.<br />
5. Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR,<br />
Northover JMA, et al. Once-only flexible sigmoidoscopy screening<br />
in prevention of colorectal cancer: a multicentre randomised<br />
controlled trial. The Lancet. 2010;375(9726):1624–33.<br />
6. Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, et<br />
al. Onceonly sigmoidoscopy in colorectal cancer screening: followup<br />
findings of the Italian randomized controlled trial–SCORE. J Natl<br />
Cancer Inst. 2011;103(17):1310–22.<br />
7. Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T,<br />
Laiyemo AO, et al. Colorectal-cancer incidence and mortality<br />
with screening flexible sigmoidoscopy. N Engl J Med.<br />
2012;366(25):2345–57.<br />
8. Holme O, Loberg M, Kalager M, Bretthauer M, Hernan MA, Aas<br />
E, et al. Effect of flexible sigmoidoscopy screening on colorectal<br />
cancer incidence and mortality: a randomized clinical trial. JAMA.<br />
2014;312(6):606–15.<br />
9. Atkin W, Wooldrage K, Parkin DM, Kralj-Hans I, MacRae E, Shah<br />
U, et al. Long term effects of once-only flexible sigmoidoscopy<br />
screening after 17 years of follow-up: the UK flexible<br />
sigmoidoscopy screening randomised controlled trial. Lancet.<br />
2017;389(10076):1299–311.<br />
10. Rutter MD, Rees CJ. Quality in gastrointestinal endoscopy.<br />
Endoscopy. 2014;46(6):526–8.<br />
11. Rex DK. Polyp detection at colonoscopy: endoscopist<br />
and technical factors. Best Pract Res Clin Gastroenterol.<br />
2017;31(4):425–33.<br />
12. Kaminski MF, Regula JR, Kraszewska E, Polkowski M,<br />
Wojciechowska U, Didkowska J, et al. Quality indicators for<br />
colonoscopy and the risk of interval cancer. N Engl J Med.<br />
2010;362:1795–803.<br />
13. Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA,<br />
et al. Adenoma detection rate and risk of colorectal cancer and<br />
death. N Engl J Med. 2014;370(14):1298–306.<br />
14. Bevan R, Blanks R, Nickerson C, Saunders B, Stebbing J, Tighe<br />
R, et al. Factors affecting adenoma detection rate in a national<br />
flexible sigmoidoscopy screening programme: a retrospective<br />
analysis. Lancet Gastroenterol Hepatol. 2019;4(3):239–47.<br />
15. Atkin W, Rogers P, Cardwell C, Cook C, Cuzick J, Wardle J, et al.<br />
Wide variation in adenoma detection rates at screening flexible<br />
sigmoidoscopy. <strong>Gastroenterology</strong>. 2004;126(5):1247–56.<br />
16. Pinsky PF, Schoen RE, Weissfeld JL, Kramer B, Hayes RB,<br />
Yokochi L; PLCO Project Team. Variability in flexible sigmoidoscopy<br />
performance among examiners in a screening trial. Clin<br />
Gastroenterol Hepatol. 2005;3(8):792–7.<br />
17. Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut<br />
AI. The impact of suboptimal bowel preparation on adenoma miss<br />
rates and the factors associated with early repeat colonoscopy.<br />
Gastrointest Endosc. 2011;73(6):1207–14.<br />
18. Issa IA, Noureddine M. Colorectal cancer screening: An<br />
updated review of the available options. World J Gastroenterol.<br />
2017;23(28):5086–96.<br />
19. Rex DK. Colonoscopic withdrawal technique is associated with<br />
adenoma miss rates. Gastrointest Endosc. 2000;51:3.<br />
20. Rees CJ, Thomas Gibson S, Rutter MD, Baragwanath P, Pullan<br />
R, Feeney M, et al. UK key performance indicators and quality<br />
assurance standards for colonoscopy. Gut. 2016;65(12):1923–9.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
16 17
FEATURE<br />
FEATURE<br />
FEATURE<br />
21. Atkin WS, Cuzick J, Northover JM, Whynes DK. Prevention<br />
16. Sivero, L. et al. Endoscopic diagnosis and treatment of<br />
of colorectal cancer by once-only sigmoidoscopy. Lancet.<br />
neuroendocrine<br />
1993;341(8847):736–40.<br />
tumors of the digestive system. Open Med. 11(1),<br />
22.<br />
369–373.<br />
Percy C,<br />
https://doi.org/10.1515/med-2016-0067<br />
Holten VV, Muir CS. International classification<br />
(2016).<br />
of<br />
17. Witteman, diseases for B. J., oncology. Janssens, Percy A. C, R., Holten Griffi oen, V, Muir G. & C, Lamers, editors, C. 2nd. B.<br />
Villous Geneva: tumours World of Health the duodenum. Organization; An 1990. analysis of the literature with<br />
23. emphasis Thomas-Gibson on malignant S, Rogers transformation. P, Cooper S, Neth. Man J. R, Med. Rutter 42, MD, 5 (1993).<br />
Suzuki N, et al. Judgement of the quality of bowel preparation at<br />
18. Levine, J. A., Burgart, L. J., Batts, K. P. & Wang, K. K. Brunner’s<br />
screening flexible sigmoidoscopy is associated with variability in<br />
gland hamartomas: Clinical presentation and pathological features<br />
adenoma detection rates. Endoscopy. 2006;38(5):456–60.<br />
of 27 cases. Am. J. Gastroenterol. 90, 290–294 (1995).<br />
24. Song M, Emilsson L, Roelstraete B, Ludvigsson JF. Risk<br />
19. Noguchi, of colorectal H. et cancer al. Prevalence in first degree of Helicobacter relatives of pylori patients infection with rate<br />
in colorectal heterotopic polyps: gastric nationwide mucosa in case-control histological study analysis in Sweden. of duodenal BMJ.<br />
specimens 2021;373:n877. from patients with duodenal ulcer. Histol. Histopathol.<br />
25. 35(2), Fracchia 169–176. M, Senore https://doi.org/10.14670/HH-18-142 C, Armaroli P, Ferraris R, Placido RD, (2020) Musso<br />
(Epub A, et 2019 al. Assessment Jul 2). of the multiple components of the variability<br />
in the adenoma detection rate in sigmoidoscopy screening, and<br />
20. Singhal, S. et al. Anorectal gastrointestinal stromal tumor: A case<br />
lessons for training. Endoscopy. 2010;42(6):448–55.<br />
report and literature review. Case Rep. Gastrointest. Med. 2013,<br />
26. Froehlich F, Wietlisbach V, Gonvers J-J, Burnand B, Vader<br />
934875 (2013).<br />
J-P. Impact of colonic cleansing on quality and diagnostic<br />
21. Modlin, yield of I. colonoscopy- M., Lye, K. D. the & Kidd, European M. A panel 5-decade of appropriateness<br />
analysis of 13,715<br />
carcinoid of gastrointestinal tumors. Cancer endoscopy 97, 934–959 European (2003). multicenter study.<br />
22. Bulur, Gastrointest A. et al. Endosc. Polypoid 2005;61(3):378–84.<br />
lesions detected in the upper<br />
27.<br />
gastrointestinal<br />
Fritz CDL, Smith<br />
endoscopy:<br />
ZL, Elsner<br />
A<br />
J,<br />
retrospective<br />
Hollander T, Early<br />
analysis<br />
D, Kushnir<br />
in 19560<br />
V.<br />
Prolonged cecal insertion time is not associated with decreased<br />
patients, a single-center study of a 5-year experience in Turkey.<br />
adenoma detection when a longer withdrawal time is achieved.<br />
N. Clin. Istanb. 8(2), 178–185. https://doi.org/10.14744/<br />
Dig Dis Sci. 2018;63(11):3120–5.<br />
nci.2020.16779 (2020).<br />
28. von Renteln D, Robertson DJ, Bensen S, Pohl H. Prolonged cecal<br />
23. Kostiainen, insertion time S., Teppo, is associated L. & Virkkula, with decreased L. Papilloma adenoma of the detection.<br />
oesophagus. Gastrointest Report Endosc. of 2017;85(3):574–80.<br />
a case. Scand. J. Thorac. Cardiovasc.<br />
29. Surg. Yang 7(1), MH, 95–97. Cho J, https://doi.org/10.3109/14017437309139176<br />
Rampal S, Choi EK, Choi Y-H, Lee JH, et al. The<br />
(1973). association between cecal insertion time and colorectal neoplasm<br />
24. Mandard,<br />
detection.<br />
A.<br />
BMC<br />
M. et<br />
Gastroenterol.<br />
al. Cancer of<br />
2013;13(1):124.<br />
the esophagus and associated<br />
30. Wong MCS, Ching JYL, Chan VCW, Lam TYT, Luk AKC, Tang<br />
lesions: Detailed pathologic study of 100 esophagectomy<br />
RSY, et al. Determinants of bowel preparation quality and its<br />
specimens. Hum. Pathol. 15, 660 (1984).<br />
association with adenoma detection: a prospective colonoscopy<br />
25. Levine, study. J. Medicine A., Burgart, (Baltimore). L. J., Batts, 2016;95(2):e2251.<br />
K. P. & Wang, K. K. Brunner’s<br />
31. gland Kim WH, hamartomas: Cho YJ, Park Clinical JY, presentation Min PK, Kang and JK, pathological Park IS. Factors features<br />
of affecting 27 cases. insertion Am. J. time Gastroenterol. and patient 90(2), discomfort 290–294 during (1995). colonoscopy.<br />
26. Ma, Gastrointest M. X. & Bourke, Endosc. M. 2000;52(5):600–5.<br />
J. Management of duodenal polyps. Best<br />
Pract. Res. Clin. Gastroenterol. 31(4), 389–399 (2017).<br />
32. Adebogun AO, Berg CD, Laiyemo AO. Concerns and challenges<br />
Author contributions<br />
in flexible sigmoidoscopy screening. Colorectal Cancer.<br />
Ç.E., 2012;1(4):309–19.<br />
M.Y.: conception, design, supervision, materials, data collection<br />
33. and processing, Doria-Rose analysis VP, Newcomb and interpretation, PA, Levin TR. literature Incomplete review, screening writer<br />
and critical flexible review. sigmoidoscopy D.A., B.Y., associated K.K. O.C., with İ.T., H.T.K.: female sex, materials, age, and data<br />
increased risk of colorectal cancer. Gut. 2005;54(9):1273–8.<br />
collection and processing, analysis and interpretation, literature review<br />
34. Laiyemo AO, Doubeni C, Pinsky PF, Doria-Rose VP, Sanderson<br />
and manuscript AK 2nd, Bresalier supervision. R, et al. Factors associated with inadequate<br />
colorectal cancer screening with flexible sigmoidoscopy. Cancer<br />
Competing Epidemiol. interests 2012;36(4):395–9.<br />
35. Cross AJ, Robbins EC, Saunders BP, Duffy SW, Wooldrage K.<br />
The authors declare no competing interests.<br />
Higher adenoma detection rates at screening associated with<br />
lower long-term colorectal cancer incidence and mortality. Clin<br />
Additional Gastroenterol information Hepatol. 2022;20(2):e148–67.<br />
36. Correspondence Brenner H, Chang-Claude and requests for J, materials Seiler CM, should Hoffmeister be addressed M. Interval to Ç.E.<br />
cancers after negative colonoscopy: population-based casecontrol<br />
study. Gut. 2012;61(11):1576–82.<br />
37. Reprints Kaminski and permissions MF, Thomas-Gibson information S, Bugajski is available M, Bretthauer at M,<br />
www.nature.com/reprints.<br />
Rees CJ, Dekker E, et al. Performance measures for lower<br />
gastrointestinal endoscopy: a European Society of Gastrointestinal<br />
Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy.<br />
Publisher’s note <strong>Spring</strong>er Nature remains neutral with regard to<br />
2017;49(4):378–97.<br />
38. jurisdictional Power S, claims Wooldrage in published K, Cross maps A. and P190 institutional The impact affi of liations. endoscopist<br />
performance on distal adenoma detection and colorectal cancer<br />
Open incidence. Access This Gut. article 2022;71(Suppl is licensed under 1):A133. a Creative Commons<br />
Attribution 4.0 International License, which permits use, sharing,<br />
Publisher’s Note<br />
adaptation, distribution and reproduction in any medium or format, as<br />
long as you give appropriate credit to the original author(s) and the source,<br />
<strong>Spring</strong>er provide a Nature link to the remains Creative neutral Commons with regard licence, to and jurisdictional indicate if claims changes in<br />
published were made. maps The images and institutional or other third affiliations. party material in this article are<br />
included in the article’s Creative Commons licence, unless indicated<br />
otherwise in a credit line to the material. If material is not included in the<br />
article’s Creative Commons licence and your intended use is not permitted<br />
by statutory regulation or exceeds the permitted use, you will need to<br />
obtain permission directly from the copyright holder. To view a copy of this<br />
licence, visit http:// creat iveco mmons. org/ licen ses/ by/4. 0/.<br />
© The Author(s) 2023<br />
C<br />
M<br />
Y<br />
CM<br />
MY<br />
CY<br />
CMY<br />
K<br />
Network with peers from across the country and share best practice<br />
Contributes towards CPD hours<br />
Free educational resources<br />
Training designed and delivered by IBD nurses for IBD nurses<br />
WHY NOT WRITE FOR US?<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
<strong>Gastroenterology</strong> <strong>Today</strong> welcomes the submission of<br />
clinical papers and case reports or news that<br />
you feel will be of interest to your colleagues.<br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com<br />
GASTROENTEROLOGY TODAY - SUMMER 2023<br />
IBD Nurse<br />
Education Modules<br />
Join us on a fully funded,<br />
innovative and practical<br />
training course.<br />
IBD STARS Event<br />
An annual event<br />
recognising excellence<br />
in improving IBD<br />
patient care.<br />
Kickstart your LOGIC Education journey…<br />
logic-tillotts.co.uk<br />
Tillotts LOGIC Education is organised and fully funded by Tillotts Pharma UK,<br />
as a part of our commitment to reinvest in the NHS and improve patient care.<br />
eLearning Portal<br />
Bite-sized, interactive<br />
educational modules for<br />
healthcare professionals<br />
in IBD.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
18 11<br />
PU-01608 | January <strong>2024</strong> Intended for UK healthcare professionals only. Some of this education may contain reference to Tillotts products. 19
FEATURE<br />
FEATURE<br />
Discover the future of healthcare at your<br />
doorstep with our all-new direct to patient<br />
kit delivery for the IBDoc ® calprotectin<br />
self-testing system. This solution empowers<br />
both patients and clinicians, redefining<br />
IBD management.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
f<br />
f<br />
f<br />
f<br />
Revolutionise<br />
Your IBD<br />
Management:<br />
IBDoc ®<br />
Calprotectin<br />
Home Test with Swift,<br />
Direct to Patient<br />
Delivery<br />
Removes requirement for administrative logistics<br />
in sending kits to patients<br />
Improves shelf life and stock level management<br />
Tracked deliveries:<br />
Z<br />
Z<br />
Weekly reports of dispatch requests available<br />
Billing only when kits are sent out<br />
Flexible delivery options (number of kits<br />
and location)<br />
02380 483000 • sales@alphalabs.co.uk • www.alphalabs.co.uk<br />
Elevate Your<br />
Healthcare Approach<br />
with Seamless Remote<br />
Testing using the<br />
BUHLMANN IBDoc ® :<br />
f<br />
f<br />
f<br />
f<br />
Excellent correlation with laboratory<br />
tests and endoscopic findings<br />
Swift quantitative testing, giving<br />
results the same day for the<br />
clinical team and the patient<br />
Minimised pre-analytical<br />
errors thanks to simple<br />
sample preparation<br />
Individualised kit containing<br />
everything the patient needs<br />
for a single test<br />
THE COST OF ILLNESS ANALYSIS OF<br />
INFLAMMATORY BOWEL DISEASE<br />
Majid Pakdin, Leila Zarei, Kamran Bagheri Lankarani and Sulmaz Ghahramani *<br />
Pakdin et al. BMC <strong>Gastroenterology</strong> (2023) 23:21 https://doi.org/10.1186/s12876-023-02648-z<br />
RESEARCH<br />
Abstract<br />
Background Inflammatory bowel disease (IBD) is a chronic<br />
inflammatory condition involving individuals across all age groups.<br />
Recent data suggests the increase in the prevalence of IBD and the<br />
surge in applying the biologic drugs in which both change the cost of<br />
IBD in recent years. Comprehensive assessment of direct and indirect<br />
cost profiles associated with IBD in our area is scarce. This study<br />
aimed to determine the economic burden of IBD in Iran from a societal<br />
perspective, using cost diaries.<br />
Methods Patients available on clinic registry and hospital information<br />
system (HIS), who were diagnosed with IBD, were invited to take<br />
part in this study. Demographic and clinical data, the healthcare<br />
resource utilization or cost items, absenteeism for the patients and<br />
their caregivers were obtained. The cost of the used resources were<br />
derived from national tariffs. The data regarding premature mortality in<br />
IBD patients was extracted from HIS. Productivity loss was estimated<br />
based on the human capital method. Then, cost date were calculated<br />
as mean annual costs per patient.<br />
Results The cost diaries were obtained from 240 subjects (Ulcerative<br />
colitis: n = 168, Crohn’s disease, n = 72). The mean annual costs per<br />
patient were 1077 US$ (95% CI 900–1253), and 1608 (95% CI 1256,<br />
1960) for the patients with ulcerative colitis and Crohn’s disease,<br />
respectively. Of the total costs, 58% and 63% were in terms of the<br />
indirect costs for the patients with ulcerative colitis and Crohn’s<br />
disease, respectively. The cost of illness for country was found to be<br />
22,331,079 US$ and 15,183,678 US$ for patients with ulcerative colitis<br />
and Crohn’s disease, respectively. Highest nationwide economic<br />
burden of IBD was found for patients older than 40 years were<br />
estimated to be 8,198,519 US$ and 7,120,891 US$, for ulcerative colitis<br />
and Crohn’s disease, respectively.<br />
Conclusion The medication was found to be the greatest contributor<br />
of direct medical costs. Productivity loss in terms of long-term disability<br />
and premature mortality were major components of IBD’s economic<br />
burden in Iran.<br />
Keywords Inflammatory bowel disease, Crohn’s disease, Ulcerative<br />
colitis, Cost of illness, Health economics<br />
Introduction<br />
*Correspondence:<br />
Sulmaz Ghahramani<br />
suli.ghahraman@gmail.com<br />
Health Policy Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran<br />
Ulcerative colitis (UC) and Crohn’s disease (CD) are included in the<br />
spectrum of disorder defined as inflammatory bowel disease (IBD),<br />
the relapsing–remitting and chronic inflammatory condition in which<br />
the gastrointestinal tract is affected [1]. IBD has lower prevalence<br />
compared to other common gastrointestinal-related disorders, such<br />
as irritable bowel syndrome, gastroesophageal reflux, and colorectal<br />
cancer; however, it is one of the gastrointestinal-related disorders<br />
with the most economic burden [1]. Data from many countries,<br />
including India [2], China [3], Scotland [4], and Turkey [5] showed an<br />
unprecedented growth of IBD worldwide. Concurrently, the incidence<br />
of the disease is increasing in Asia [6] and Iran [7]. Low mortality of the<br />
disease, the diagnosis at early ages, and its chronic nature have driven<br />
this increase in the disease’s prevalence. Using biological medications<br />
in the treatment of IBD changed the need for hospitalization and<br />
surgeries and also changed the cost of the disease in recent years. So,<br />
these highlight the importance of the economic burden evaluation of<br />
the disease [8, 9]. As stated before, in Iran, the IBD incidence is rising<br />
while information on its cost is scarce. Two studies have evaluated<br />
direct medical cost and hospitalization cost of the disease [10, 11].<br />
Nevertheless, health policy makers should have reliable information<br />
regarding the cost of illness to quantify the impact of the disease on a<br />
society. This can inform healthcare cost projection, as well as resource<br />
allocation [12]. Regarding the mentioned points, it is necessary to<br />
assess the cost of IBD, including direct medical costs, direct nonmedical<br />
costs, and indirect costs to determine the economic burden<br />
of IBD in Iran. In our study, we aim to evaluate the cost of IBD in a<br />
multicenter setting.<br />
Methods<br />
Participants and data collection<br />
This cost of illness analysis was conducted on the patients diagnosed<br />
with IBD in 2021. For data collection, we used the phone records<br />
available in Shiraz’ hospital information systems (HIS) and IBD clinic<br />
at Faghihi hospital, which is referral IBD clinic affiliated to the Shiraz<br />
University of Medical Sciences. Using the convenient sampling method,<br />
patients were invited to take part the study through phone calls. Then,<br />
the data was obtained through a face-to-face interview while the<br />
patients were referred to the clinic. This clinic and referral hospitals<br />
all provide the care to the IBD patients, mostly from Fars province<br />
and sometimes from neighboring provinces. Our study as a partial<br />
economic evaluation technique, aimed to calculate the total costs of IBD<br />
As pioneers in innovative healthcare<br />
Elevate your IBD management today<br />
solutions, we're proud to announce our<br />
Reach out to us at digestivedx@alphalabs.co.uk<br />
enhanced approach to home testing logistics.<br />
20 21<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong>
FEATURE<br />
Pakdin et al. BMC <strong>Gastroenterology</strong> (2023) 23:21<br />
FEATURE<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
in Iran from the society perspective. The IBD diagnosis was confirmed<br />
based on clinical, endoscopic, and histological criteria, as described<br />
elsewhere [13]. Demographic data (including sex, age, marital status,<br />
educational level, educational level, income, and hours of paid work),<br />
clinical data (including disease duration, disease progression, and<br />
extra-intestinal involvement) were obtained from all participants, and they<br />
were interviewed to fill out the cost diary. The disease progression was<br />
defined by calculating Mayo score and Crohn’s disease activity index<br />
(CDAI) in patients with UC, and CD, respectively. To obtain an estimated<br />
prevalence of IBD in Fars province, the population ratio of Fars province<br />
in Iran was multiplied by an estimated prevalence of IBD in Iran in 2021<br />
[14, 15], which found to be 1800. The sample volume was determined<br />
to be 233 patients for estimation of costs of illness based on 95% CI,<br />
margin error of 6%, and the estimated prevalence of IBD in Fars province<br />
(http://www.raosoft.com/samplesize.html).<br />
Cost diary<br />
Cost diary is an instrumental method which is developed by Goosens<br />
et al. and is used to estimate the cost of a condition. Since no<br />
significant difference has been found among the patients’ report and<br />
medical records, there is no need to check medical reports when the<br />
cost diary is used [16].<br />
In order to prevent recall bias, the number of physician visits,<br />
physiotherapy, purchased drugs dosage, and all other related disease<br />
‘s costs during 3-month prior to the interview were asked from<br />
patients, which were scaled up by a factor of four to extrapolate to<br />
the mean number of each item during 1-year. Only for hospitalization<br />
and surgeries, the duration of 1- year was considered in cost diary.<br />
To minimize missing information and partial responses, telephone<br />
contacts were made after the initial visit, whenever it was needed.<br />
We had a protocol for phone calls. We made phone calls, in case of<br />
none- response we considered maximum number of three phone calls<br />
in different hours of day and variable days of week to make telephone<br />
contacts. Diary records were used to estimate resource used. The cost<br />
of the used resources were derived from national tariffs. The costs<br />
were recorded in Rial and then we converted to US dollars based<br />
on the moving average of the exchange rate in the 2021 252,000:1<br />
(Rial:US) (Additional file 1: Table S1) .<br />
Death registration database<br />
We extracted death data of patients with IBD, who were died because<br />
of IBD-related causes, from HIS during 2013–2021 to estimate<br />
economic burden of premature mortality for UCCD.<br />
Direct medical costs<br />
Direct medical costs included physician visits, physiotherapy sessions,<br />
nutrition sessions, purchased medication, surgeries, and para-clinical<br />
tests. The cost of each item expected on medication was estimated<br />
by the mean number of each item, multiplied by the contemporary<br />
tariff of the Ministry of Health and Medical Education (MoHME). The<br />
contemporary tariff is defined for each unit of healthcare service item<br />
by the MoHME annually in two forms of private and public services.<br />
In our study, we used the weighted average of these two forms based<br />
on the last national utilization survey [17]. The cost of purchased<br />
medication was calculated by the number of each item, multiplied by<br />
the unit cost of each item defined by Food and Drug Administration<br />
(http://irc.fda.gov.ir/nfi#).<br />
Direct nonmedical costs<br />
Transportation, self-help group, hours of paid household help, and<br />
goods-related to the condition (including alternative medicine, assistive<br />
devices, special diet, and books) were considered the components of<br />
direct nonmedical costs. Transportation cost was obtained from the<br />
sum of public and personal transportation. Public transportation cost<br />
was calculated based on the mean number of public transportation<br />
service use multiplied by the tariff of transportation defined by<br />
Municipal. Personal transportation and other items were calculated<br />
based on the real reported costs by patients in the cost diary.<br />
Indirect costs<br />
The human capital approach was used to estimate indirect costs.<br />
Productivity losses because of disability and premature mortality were<br />
considered components of indirect costs in our study. We divided<br />
disability into short- and long-term disability.<br />
Productivity losses due to short‐term disability<br />
Productivity losses in terms of short-term disability were obtained from<br />
temporary absenteeism from work for patients with the disease, and<br />
the caregivers. To calculate productivity loss because of temperament<br />
absenteeism of patients from work for therapy appointments, the<br />
hourly wage of lowest-paid unskilled government workers (LPUGW) of<br />
the Ministry of Labor was multiplied by the number of absence hours<br />
for each patient. For calculation of the productivity loss because of<br />
absenteeism of the caregiver the number of hospitalization days for the<br />
patients who were hospitalized during 1-year was added to 14 days<br />
per hospitalization. Then, the calculated number was multiplied by the<br />
LPUGW. It was assumed that work productivity loss was experienced<br />
by caregivers during hospitalization days to fulfill the responsibility<br />
of caregiving.<br />
Productivity losses due to long‐term disability<br />
Early retirement of patients, permanent absenteeism from work<br />
because of disability, and unpaid household work for caregivers of<br />
disabled patients were considered estimating productivity losses<br />
due to long-term disability. To estimate the cost of early retirement,<br />
the amount of lost pension, compared to the full pension, was<br />
calculated for any patient who had early retired. To calculate the cost of<br />
permanent absenteeism from work, the annual wage of LPUGW was<br />
considered for patients who completely could not work, and were not<br />
paid a defined benefit pension. To estimate unpaid household work for<br />
caregivers of disabled patients, the number of patients who could not<br />
take care of themselves were multiplied by the annual wage of LPUGW<br />
because of unpaid permanent caregiver responsibility.<br />
Premature mortality<br />
Standard expected years of life lost (SEYLL) was used to predict the<br />
productivity loss due to premature mortality. We used the following<br />
formula to estimate SEYLL [18]:<br />
SEYLL = N × L x<br />
where N identifies the number of deaths at a certain age, and L x<br />
refers<br />
to the remaining life-expectancy at the age of death. Based on many<br />
arguments and the last update of global health estimates by WHO, we<br />
decided not to take into account time discounting and age-weighting<br />
[19–22]. We obtained mean number of annual deaths by age, gender,<br />
and type of disease. The gender-specific remaining life-expectancy<br />
at the age of death was calculated based on the 2020 Iran lifetable<br />
from World Health Organization [23], and subsequently SEYLL for<br />
each disease was calculated by the sum of the two obtained genderspecific<br />
SEYLL for the type of disease. Then, the calculated SEYLL<br />
was multiplied by gross domestic production (GDP) per capita for Iran,<br />
which is defined by World Bank [24], to estimate the total economic<br />
burden of premature mortality for each disease. To obtain the mean<br />
cost per patient, the total cost of premature mortality was divided by<br />
the estimated prevalence of the disease in Fars Province.<br />
Statistical analysis<br />
All statistical analyses were performed using SPSS 26.0. Mean, and<br />
the standard deviation was used to present continuous variables,<br />
while categorical variables were shown as frequency and percentage.<br />
Costs were reported as mean costs with 95% CI estimated using nonparametric<br />
boodstrap sampling.<br />
Results<br />
Demographic characteristics<br />
Among 286 patients who were initially invited, 240 patients accepted to<br />
take part. The mean ± Standard deviation (SD) age of participants was<br />
41 ± 13 and 39 ± 14 for UC and CD, respectively. Majority of patients<br />
were married, and reside in the cities. The gender was approximately<br />
equal between males and females in the both diseases, and UC was<br />
predominant disease among participants. Descriptive results based<br />
on gender, marital status, employment status, disease type, and other<br />
variables are summarized in Table 1.<br />
Direct medical costs<br />
Cost items are categorized in Table 2. Number [%] of participants using<br />
resources, and mean annual cost for each category are shown in the<br />
table for UC and CD, separately.<br />
Drugs, which were taken by patients, are classified into six categories<br />
(Table 3). Number [%] of participants taking each medication’s type,<br />
and mean annual cost for each type are shown in the table based on<br />
the disease type.<br />
Medication had the greatest share of direct medical costs in both<br />
types of IBD, accounting for 31.6% and 23.0% of the total costs in<br />
the patients with UC and CD, respectively (445.52$ per patient in<br />
UC, and 586.96$ per patient in CD). Among types of medication,<br />
aminosalicylates contributed to the most prescription proportion in<br />
both diseases (66.2% in UC and 63.9% in CD). It shared the highest<br />
cost of medication types in patients with UC, accounting for 244.63$<br />
per patient. Less than twenty percent of patients consumed biological<br />
agent; however, this type of medication accounted for the highest and<br />
the second highest medication costs in patients with CD (235.11$),<br />
and UC (100.72$), respectively (Table 3). Hospitalization was another<br />
important contributor of direct medical costs, especially in the patients<br />
with CD, responsible for 10% of the total costs (161.29$) per patient.<br />
Physiotherapy and nutrition consult almost did not impose cost to the<br />
studied IBD patients (Table 2).<br />
Direct nonmedical costs<br />
Although the proportion of using nonmedical resources were high by<br />
Table 1 Patients’ characteristics<br />
Characteristic<br />
SD, standard deviation<br />
Ulcerative<br />
colitis<br />
(n = 168)<br />
Age, mean (SD), years 40.64 (12.90) 39.11 (14.04)<br />
Age groups<br />
Sex<br />
0–29 34 [20.2] 19 [26.4]<br />
30–39 55 [32.7] 18 [25.0]<br />
≥ 40 79 [47.0] 35 [48.6]<br />
Female 81 [48.2] 30 [41.7]<br />
Male 87 [51.8] 42 [58.3]<br />
Marital status<br />
Single 36 [21.4] 25 [34.7]<br />
Married 125 [74.4] 43 [59.7]<br />
Widowed 5 [3] 0<br />
Divorced 2 [1.2] 4 [5.6]<br />
Employment status<br />
Non-employed 81 [48.2] 40 [55.6]<br />
Employed 70 [41.7] 22 [30.6]<br />
Disabled 8 [4.8] 7 [9.7]<br />
Retired 9 [5.4] 3 [4.2]<br />
Educational level<br />
Illiterate 21 [12.5] 7 [9.7]<br />
Diploma 87 [51.8] 42 [58.3]<br />
Bachelor degree or higher 60 [35.7] 23 [32]<br />
Residence<br />
City 136 [81.0] 54 [75]<br />
Village 32 [19.0] 18 [25]<br />
Disease duration, years 9.49 (7.98) 7.86 (6.69)<br />
Disease progression<br />
Continuously active 44 [26.2] 15 [20.8]<br />
Intermittently active 81 [48.2] 41 [56.9]<br />
Inactive 43 [25.6] 16 [22.2]<br />
Extra-intestinal involvement<br />
Liver involvement 23 [13.7] 13 [18.1]<br />
Oral involvement 38 [22.6] 18 [25.0]<br />
Skin involvement 37 [22.0] 14 [19.4]<br />
Vertebra involvement 12 [7.1] 11 [15.3]<br />
Eye involvement 42 [25.0] 21 [29.2]<br />
Joint involvement 59 [35.1] 30 [41.7]<br />
Lung involvement 5 [3.0] 3 [4.2]<br />
Fistula 4 [2.4] 14 [19.4]<br />
Data are presented as n [%] or mean (SD)<br />
Crohn’s disease (n = 72)<br />
Direct medical costs<br />
Cost items are categorized in Table 2. Number [%] of<br />
participants using resources, and mean annual cost for<br />
each category are shown in the table for UC and CD,<br />
separately.<br />
Drug<br />
six cate<br />
ing eac<br />
type ar<br />
Med<br />
cal cos<br />
23.0%<br />
respect<br />
patient<br />
icylates<br />
in both<br />
the hig<br />
accoun<br />
percen<br />
this ty<br />
the sec<br />
(235.11<br />
pitaliza<br />
medica<br />
sible fo<br />
Physio<br />
impose<br />
Direct n<br />
Althou<br />
were h<br />
less tha<br />
patient<br />
mostly<br />
Indirec<br />
Indirec<br />
respon<br />
patient<br />
tivity l<br />
frequen<br />
due to<br />
formed<br />
for pat<br />
of prod<br />
in UC<br />
large s<br />
and 43<br />
from w<br />
ity loss<br />
types (<br />
in CD<br />
was an<br />
accoun<br />
eases (<br />
in CD)<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
22 23
Pakdin et al. BMC <strong>Gastroenterology</strong> (2023) 23:21<br />
FEATURE<br />
Table 2 Resource utilization and costs per patient<br />
Cost categories<br />
Participants using resources,<br />
n [%]<br />
Page 5 of 8<br />
Mean annual costs in US$ (SD) [% of total costs]<br />
UC (n = 168) CD (n = 72) UC (n = 168) CD (n = 72)<br />
Total costs 168 [100.0] 72 [100.0] 1076.89 (1159.14) [100.0] 1608.24 (1497.11) [100.0]<br />
Direct medical cost 157 [93.5] 72 [100.0] 445.52 (691.76) [41.4] 586.96 (629.74) [36.5]<br />
Physician visit 131 [78.0] 68 [94.4] 15.93 (31.52) [1.5] 21.56 (27.34) [1.3]<br />
General physician 12 [7.1] 7 [9.7] 1.24 (7.82) [0.1] 2.16 (9.63) [0.1]<br />
Specialist 14 [8.3] 3 [4.2] 1.60 (11.73) [0.1] 0.38 (2.13) [0.0]<br />
Subspecialist 123 [73.2] 66 [91.7] 12.87 (16.19) [1.2] 18.81 (25.04) [1.2]<br />
Psychiatrist 4 [2.4] 2 [2.8] 0.21 (1.45) [0.0] 0.20 (1.18) [0.0]<br />
Physiotherapy 4 [2.4] 0 [0.0] 0.13 (1.01) [0.0] 0.00 (0.00) [0.0]<br />
Nutritionist consult 0 [0.0] 0 [0.0] 0.00 (00.00) [0.0] 0.00 (0.00) [0.0]<br />
Hospitalization 24 [14.3] 25 [34.7] 64.64 (251.35) [6.0] 161.29 (380.94) [10.0]<br />
Para-clinic 124 [73.8] 62 [86.1] 22.93 (37.64) [2.1] 27.04 (50.00) [1.7]<br />
Laboratory 116 [69.0] 60 [83.3] 7.59 (9.93) [0.7] 10.64 (11.10) [0.7]<br />
Imaging 48 [28.6] 29 [40.3] 15.33 (31.79) [1.4] 16.41 (34.07) [1.0]<br />
Medication 152 [90.5] 69 [95.8] 340.38 (633.18) [31.6] 369.97 (441.22) [23.0]<br />
Surgeries 7 [4.2] 8 [11.1] 1.51 (7.59) [0.1] 7.10 (25.40) [0.4]<br />
Direct nonmedical cost 140 [83.3] 65 [90.3] 8.92 (36.29) [0.8] 4.67 (7.69) [0.3]<br />
Transportation<br />
Public transportation 62 [39.1] 33 [45.8] 0.42 (0.86) [0.0] 0.56 (1.21) [0.0]<br />
Personal transportation 82 [48.8] 34 [47.2] 4.80 (24.02) [0.4] 3.03 (6.36) [0.2]<br />
Medical related goods (including assistive devices, alternative<br />
medicine, special diet, related books)<br />
9 [5.4] 6 [8.3] 2.07 (18.68) [0.2] 0.98 (4.12) [0.1]<br />
Paid household help 1 [0.6] 1 [1.4] 0.09 (1.10) [0.0] 0.10 (0.84) [0.0]<br />
Self-help group 0 [0.0] 0 [0.0] 0.00 (0.00) [0.0] 0.00 (0.00) [0.0]<br />
Indirect costs 58 [34.5] 33 [45.8] 622.46 (808.61) [57.8] 1016.62 (1169.81) [63.2]<br />
Short-term disability 42 [25.0] 30 [41.7] 88.49 (221.46) [8.2] 193.00 (472.27) [12.0]<br />
Temporary absenteeism for patients 22 [13.1] 8 [11.1] 51.62 (175.87) [4.8] 88.11 (378.42) [5.5]<br />
Temporary absenteeism for caregivers 24 [14.3] 25 [34.7] 36.87 (126.52) [3.4] 104.89 (217.58) [6.5]<br />
Long-term disability 23 [13.7] 14 [19.4] 266.50 (745.37) [24.7] 436.40 (980.39) [27.1]<br />
Early retirement 7 [4.2] 12 [2.8] 42.01 (216.60) [3.9] 28.99 (201.20) [1.8]<br />
Permanent absenteeism 14 [8.3] 9 [12.5] 174.60 (580.82) [16.2] 261.91 (697.80) [16.3]<br />
Unpaid household work for caregivers of disabled patients 4 [2.4] 5 [6.9] 49.89 (320.39) [4.6] 145.50 (536.37) [9.0]<br />
Premature death – – 276.47 [24.8] 387.22 [24.1]<br />
participants, the related costs accounted for less than 1% of the total<br />
costs in both diseases (8.92$ per patient in UC, and 4.67$ per patient<br />
in CD). They were mostly driven by transportation (Table 2).<br />
Indirect costs<br />
Indirect costs were major contributors of the total costs, responsible<br />
for more than a half of the costs (622.46$ per patient in UC, and<br />
1016.62$ per patient in CD). Productivity losses induced by shortterm<br />
disability were more frequent among participants, as compared<br />
with the losses due to long-term disability, and were almost equally<br />
formed by two components of temporary absenteeism for patients<br />
and caregivers. Despite the lower frequency of productivity losses due<br />
to long-term disability (13.7% in UC patients, and 19.4% CD patients),<br />
they imposed a large share of the total costs (266.50$ per patient in<br />
UC, and 436.40$ per patient in CD). Permanent absenteeism from<br />
work was predominant compartment of productivity losses induced by<br />
long-term disability in both disease types (174.60$ per patient in UC,<br />
and 261.91$ per patient in CD). Productivity loss because of premature<br />
death was another important contributor of the total costs, accounting<br />
for almost a quarter of the costs in both diseases (276.47$ per patient<br />
in UC, and 387.22$ per patient in CD) (Table 2).<br />
Cost of illness for country<br />
The characteristics of participants, including age, sex, disease type<br />
and age at diagnosis were almost equivalent to those described for<br />
the recent pilot feasibility study of first nation-wide IBD registry in Iran,<br />
which enabled us to extrapolate the mean annual total costs regarding<br />
disease type and age group for the country [17]. An estimated<br />
prevalence of IBD in Iran in 2021 was used for the calculation [15]. The<br />
cost of illness for country was found to be 22,331,079 and 15,183,678<br />
for UC and CD, respectively (Table 4).<br />
ers to study the economic burden of specific diseases in<br />
Iran, as diagnostic data is not ordinarily coded for outpatient<br />
visits, and the patients’ resource utilization are<br />
not recorded. Thus, there is the paucity of FEATURE<br />
information<br />
on resource utilization consumed by patients with specific<br />
diseases. However, to the best of our knowledge this<br />
study among the first comprehensive studies in Iran<br />
Discussion<br />
assessing both direct and indirect cost profiles associated<br />
In<br />
with<br />
this<br />
IBD.<br />
retrospective cohort study, we aimed to determine the<br />
economic<br />
Several<br />
burden<br />
studies<br />
of IBD,<br />
have<br />
and<br />
evaluated<br />
to compare the<br />
the<br />
profiles<br />
economic<br />
of costs<br />
burden<br />
in UC<br />
and<br />
of IBD<br />
CD patients.<br />
in other<br />
There<br />
countries<br />
are barriers<br />
[25–37].<br />
to study<br />
Consistent<br />
the economic<br />
with<br />
burden<br />
their<br />
of<br />
specific<br />
findings,<br />
diseases<br />
we found<br />
in Iran,<br />
that<br />
as diagnostic<br />
the CD<br />
data<br />
patients’<br />
is not ordinarily<br />
resource<br />
coded<br />
utilization<br />
was visits, higher and than the patients’ that of resource UC patients, utilization and are not this recorded. differ-<br />
for<br />
outpatient<br />
Thus, ence there was is more the paucity considerable of information in hospitalization, on resource utilization surgeries,<br />
consumed and laboratory by patients sectors. with specific Variations diseases. in However, the pathogenesis to the best of of<br />
our the knowledge two diseases this study can is explain among the this first difference, comprehensive as UC studies might<br />
in not Iran lead assessing to irreversible both direct and and indirect systemic cost profiles damage associated observed<br />
with in CD IBD. patients [38]. However, the amount of difference<br />
among the annual mean costs of UC and CD differs from<br />
Several a study studies to another, have evaluated based the on economic the design burden and of IBD population in other<br />
countries of study, [25–37]. as inconsistencies Consistent with their are findings, created we in found the that results the CD of<br />
patients’ cost of resource illness utilization studies was when higher comparing than that of one UC patients, to another and<br />
this due difference variations was more in method considerable [30]. in Annual hospitalization, mean surgeries, costs per and<br />
laboratory patient for sectors. UC Variations and CD in were the pathogenesis between 6217–11,477 of the two diseases US$,<br />
can and explain 11,034–18,932 this difference, US$ as UC in might the not USA, lead to respectively. irreversible and The<br />
systemic corresponding damage observed ranges in were CD patients 8949–10,395 [38]. However, euros, the amount and<br />
of 2898–6742 difference among euros the in annual European mean costs countries of UC and [39, CD 40]. differs In our<br />
study, annual mean costs per patient for UC and CD<br />
from a study to another, based on the design and population of<br />
Table 4 Mean annual total costs in US$ for country<br />
Age group (year) Ulcerative colitis Crohn’s disease<br />
0–29 6,454,537 2,422,262<br />
30–39 7,678,023 5,640,525<br />
≥ 40 8,198,519 7,120,891<br />
All age groups 22,331,079 15,183,678<br />
spread<br />
IBD pa<br />
ing to a<br />
this typ<br />
of cole<br />
ings, de<br />
receive<br />
CD pat<br />
total c<br />
tance o<br />
them a<br />
ipants<br />
Consid<br />
therapy<br />
tine as<br />
IBD pa<br />
Direc<br />
the tota<br />
rect co<br />
had be<br />
patient<br />
applyin<br />
disease<br />
design<br />
for the<br />
to enro<br />
Shor<br />
disabili<br />
ity loss<br />
of the<br />
to a Ge<br />
32% an<br />
tively [<br />
produc<br />
found<br />
[33]. It<br />
role in<br />
UC: Ulcerative colitis; CD: Crohn’s disease; SD: standard deviation<br />
Table 3 Frequency and costs of purchased medication<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
Drug category Participants taking medication, n [%] Mean annual costs in US$ (SD) [% of total medication<br />
costs]<br />
Miscellaneous drug groups include analgesics, gastrointestinal associated drugs, and others<br />
UC: Ulcerative colitis; CD: Crohn’s disease; SD: standard deviation<br />
UC (n = 168) CD (n = 72) UC (n = 168) CD (n = 72)<br />
Aminosalicylates 128 [66.2] 46 [63.9] 244.63 (554.37) [71.9] 100.72 (205.08) [25.4]<br />
Biological agents 14 [8.3] 19 [26.4] 74.46 (264.97) [21.9] 235.11 (425.19) [59.3]<br />
Corton 47 [28.0] 24 [33.3] 1.86 (4.23) [0.5] 2.03 (4.36) [0.5]<br />
Immunomodulators 48 [28.6] 35 [48.6] 6.17 (22.09) [1.8] 12.96 (47.79) [3.3]<br />
Supplementary 68 [40.5] 37 [51.4] 5.25 (10.77) [1.5] 7.82 (15.49) [2.0]<br />
Psychotropic drugs 11 [6.5] 9 [12.5] 0.40 (2.05) [0.1] 0.82 (3.89) [0.2]<br />
Miscellaneous 53 [31.5] 25 [34.7] 7.60 (16.92) [2.2] 10.51 (20.19) [2.6]<br />
Publishers Comment<br />
For over 30 years thanks to trade support, we have been able to provide those working within <strong>Gastroenterology</strong> Departments<br />
and Endoscopy Units with quarterly copies of <strong>Gastroenterology</strong> <strong>Today</strong> free of charge in the knowledge that those receiving<br />
our dedicated publication enjoy having something to pick up and read during their free time. As in the current climate, return on<br />
investment appears to be the buzz word amongst suppliers, we would appreciate you mentioning <strong>Gastroenterology</strong> <strong>Today</strong><br />
when enquiring about products advertised.<br />
In respect of this current issue we would like to thank the following companies for their advertising support as without their<br />
contribution towards our print and postal costs this issue would not have been published. Alpha Laboratories, Biohit, Steris,<br />
Infai, Micro Tech, Nordic, Tillots.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
24 25
FEATURE<br />
FEATURE<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
study, as inconsistencies are created in the results of cost of illness<br />
studies when comparing one to another due variations in method<br />
[30]. Annual mean costs per patient for UC and CD were between<br />
6217–11,477 US$, and 11,034–18,932 US$ in the USA, respectively.<br />
The corresponding ranges were 8949–10,395 euros, and 2898–6742<br />
euros in European countries [39, 40]. In our study, annual mean<br />
costs per patient for UC and CD were found to be 1077 US$ (95% CI<br />
900–1253), and 1068 (95% CI 1256, 1960) respectively, which is in line<br />
with the results of studies in UK and Germany in terms of CD: UC costs<br />
ratio [25, 34]. These two studies have applied a similar method of using<br />
patient-reported resource utilization to estimate economic burden of<br />
the disease (bottom-up approach). The difference of total costs per<br />
patient between Iran and European countries could be explained by<br />
the fluctuations in Iran’s currency exchange rate to dollar in the last<br />
few years.<br />
According to our findings, the medication use represents the major<br />
source of direct medical costs (76.4% and 63.0% of direct medical<br />
costs in UC and CD patients), while costs related to surgery and<br />
hospitalization have not a large share of direct cost. The results of more<br />
recent studies are consistent with our results [30–33]. The widespread<br />
application of biological agents in the treatment of IBD patients has<br />
changed the healthcare outlook, leading to a substantial shift in cost<br />
profiles [33]. The effect of this type of medication was significant<br />
on the reduction of colectomy in UC patients [41–43]. According to<br />
findings, despite the relatively low proportion of patients who received<br />
biological agents (8.3% and 26.4% of UC and CD patients); they<br />
shaped a significant contributor of the total costs in both diseases. This<br />
highlights the importance of better insurance coverage for such drugs<br />
to make them affordable. According to results, none of the participants<br />
used the nutritional consultation and assessment. Considering the<br />
importance of supportive nutritional therapy in the clinical care of IBD<br />
patients [44, 45], routine assessment and monitoring of nutritional<br />
status in IBD patients in Iran is highly encouraged.<br />
Direct nonmedical costs were minor contributors to the total costs,<br />
as compared with direct medical and indirect costs. Based on our<br />
findings, none of participants had been a member of self-help groups.<br />
In these groups, patients share their own experiences, which can lead<br />
to applying executable strategies for management of chronic disease<br />
by other patients [46]. Therefore, it is pivotal to design self-help groups<br />
for IBD patients in Iran which fit for their need; subsequently, they<br />
should be encouraged to enroll in such groups.<br />
Short- and long-term productivity losses because of disability<br />
were also evaluated. We found that productivity losses because<br />
of disability handled 33.0% and 39.1% of the total costs in UC<br />
and CD, respectively. According to a German study, long term<br />
productivity losses shared 32% and 49% of the total costs in UC<br />
and CD, respectively [34]. In a more recent study in the Netherlands,<br />
productivity losses due to IBD-related absenteeism were found to<br />
be 16% and 39% of the total costs, respectively [33]. It seems that<br />
biological agents have had an effective role in reducing productivity<br />
losses because of disability in CD patients. However, due to different<br />
methodologies in measurement of productivity losses due to disability,<br />
we have limitations for a more detailed comparison with the results of<br />
other studies. In our study, we considered absenteeism and unpaid<br />
household work for caregivers, which were important contributors to<br />
costs for CD patients, accounting for 6.5% and 9.0% of the total costs,<br />
respectively. We did not evaluate presenteeism in our study, since no<br />
validated questionnaire is available to evaluate presenteeism with a<br />
recall time of over 7 days [33].<br />
The premature mortality was another major contributor of costs in both<br />
diseases, which is in line with findings of systematic analysis of the<br />
global burden of inflammatory bowel disease. It shows the fact that<br />
IBD-associated premature mortality forms a great share of disease<br />
burden in countries with low socio-demographic index (SDI) [47].<br />
There is insufficient population-based data evaluating the causes of<br />
premature mortality in IBD patients in Iran. In a study assessing the<br />
trend of colectomy in the country, colorectal cancer was found to be<br />
the leading cause of death in IBD patients undergoing colectomy. In<br />
this regard, cancer screening protocols should be routinely performed<br />
in IBD patients [9]. Cancer and cardiovascular disease were found to<br />
be leading causes of mortality in IBD patients in the USA. Therefore,<br />
healthy lifestyle behaviors should be routinely assessed in IBD<br />
patients, and adherence to such lifestyle should be encouraged to<br />
reduce contributing risk for cancer and cardiovascular disease, and<br />
subsequently the risk of premature mortality and related costs in IBD<br />
patients [48].<br />
Limitations<br />
This study provided a more comprehensive view of the costs of<br />
illness for IBD in the Iran. However, this study was limited by the small<br />
sample size and prevalence approach for cost diaries. Furthermore<br />
some aspects of estimation might be affected by purchasing power of<br />
participants.<br />
Conclusion<br />
The medication was found to be the greatest contributor to direct<br />
medical costs. Productivity loss due to long-term disability and<br />
premature mortality were major components of inflammatory bowel<br />
disease burden in Iran.<br />
Supplementary Information<br />
The online version contains supplementary material available at<br />
https://doi.org/10.1186/s12876-023-02648-z.<br />
Additional file 1: Table S1. Unit Costs of Medical Resources Consumed<br />
by Patients/*: For Nurition Consult, Imaging, Laboratory, and Surgery<br />
constant of k should be multipled by given data; k = 13600.<br />
Acknowledgements<br />
We are grateful to all patients who participated in this study.<br />
Author contributions<br />
Conceptualization, Visualization, and Methodology: KBL, LZ, SG.<br />
Supervision, and Project administration: KBL, SG. Investigation: MP,<br />
SG. Writing, reviewing, and editing: MP, LZ, SG. Data curation, and<br />
Software: MP. All authors read and approved the final manuscript.<br />
Funding<br />
This study was funded by Shiraz University of Medical Sciences<br />
(SUMS).<br />
Availability of data and materials<br />
The data that support the findings of this study are available from the<br />
corresponding author upon reasonable request.<br />
Declarations<br />
Ethics approval and consent to participate<br />
Informed consent was obtained from all participants, their anonymity<br />
was guaranteed, and the study protocol was approved by approved by<br />
Ethical Committee of Shiraz University of Medical Sciences approved<br />
with Reg. No: IR.SUMS.REC.1400.637. The study protocol followed the<br />
ethical guidelines of the 2013 Declaration of Helsinki.<br />
Consent for publication<br />
Not applicable.<br />
Competing interests<br />
The authors confirm that there is no conflict of interest to declare.<br />
Received: 10 August 2022 Accepted: 10 January 2023<br />
Published online: 19 January 2023<br />
References<br />
1. Beard JA, Franco DL, Click BH. The burden of cost in<br />
inflammatory bowel disease: a medical economic perspective<br />
and the future of value-based care. Curr Gastroenterol Rep.<br />
2020;22(2):1–7.<br />
2. Desai H, Gupte P. Increasing incidence of Crohn’s disease in<br />
India: is it related to improved sanitation? Indian J Gastroenterol.<br />
2005;24:23–4.<br />
3. Zheng JJ, Zhu XS, Huangfu Z, Gao ZX, Guo ZR, Wang Z. Crohn’s<br />
disease in mainland China: a systematic analysis of 50 years of<br />
research. Chin J Dig Dis. 2005;6(4):175–81.<br />
4. Armitage E, Drummond HE, Wilson DC, Ghosh S. Increasing<br />
incidence of both juvenile-onset Crohn’s disease and ulcerative<br />
colitis in Scotland. Eur J Gastroenterol Hepatol. 2001;13(12):<br />
1439–47.<br />
5. Can G, Poşul E, Yılmaz B, Can H, Korkmaz U, Ermiş F, et al.<br />
Epidemiologic features of ınflammatory bowel disease in Western<br />
Blacksea region of Turkey for the last 10 years: retrospective<br />
cohort study. Korean J Intern Med. 2019;34(3):519.<br />
6. Hu P-J. Inflammatory bowel disease in Asia: the challenges and<br />
opportunities. Intest Res. 2015;13(3):188.<br />
7. Malekzadeh MM, Vahedi H, Gohari K, Mehdipour P, Sepanlou<br />
SG, Ebrahimi Daryani N, et al. Emerging epidemic of inflammatory<br />
bowel disease in a middle income country: a nation-wide study<br />
from Iran. Arch Iran Med. 2016;19:2–15.<br />
8. van Linschoten RCA, Visser E, Niehot CD, van Der Woude CJ,<br />
Hazelzet JA, van Noord D, et al. Systematic review: societal<br />
cost of illness of inflammatory bowel disease is increasing due to<br />
biologics and varies between continents. Aliment Pharmacol Ther.<br />
2021;54(3):234–48.<br />
9. Ghahramani S, Paparisabet M, Sayari M, Hosseini SV, Lankarani<br />
KB. Colectomy in ulcerative colitis: trends in southern iran in a<br />
decade. Arch Iran Med. 2021;24(9):665.<br />
10. Balaii H, Olfatifar M, Narab SO, Hosseini AA, Salehi AS, Shahrokh<br />
S. Estimation the direct cost of inflammatory bowel disease in<br />
Iranian patients; the one-year follow-up. Gastroenterol Hepatol<br />
Bed Bench. 2019;12(Suppl1):S87.<br />
11. Lankarani KB, Ghahramani S, Hadipour M, Pourhashemi M,<br />
Mahmoodi A, Zeraatpishe M, et al. Determinants of hospital costs<br />
of inflammatory bowel disease. Govaresh. 2019;24(4):230–7.<br />
12. Greenberg D, Ibrahim MIBM, Boncz I. What are the challenges<br />
in conducting cost-of-illness studies? Value Health Reg Issues.<br />
2014;4:115–6.<br />
13. Lennard-Jones J. Classification of inflammatory bowel disease.<br />
Scand J Gastroenterol. 1989;24(sup170):2–6.<br />
14. National Portal of Statistics: Statistical Center of Iran; 2021.<br />
https://www.amar.org.ir.<br />
15. Olfatifar M, Zali MR, Pourhoseingholi MA, Balaii H, Ghavami<br />
SB, Ivanchuk M, et al. The emerging epidemic of inflammatory<br />
bowel disease in Asia and Iran by 2035: a modeling study. BMC<br />
Gastroenterol. 2021;21(1):1–8.<br />
16. Goossens ME, Rutten-van Mölken MP, Vlaeyen JW, van der<br />
Linden SM. The cost diary: a method to measure direct and<br />
indirect costs in costeffectiveness research. J Clin Epidemiol.<br />
2000;53(7):688–95.<br />
17. Malekzadeh MM, Sima A, Alatab S, Sadeghi A, Daryani NE, Adibi<br />
P, et al. Iranian Registry of Crohn’s and Colitis: study profile of first<br />
nationwide inflammatory bowel disease registry in Middle East.<br />
Intest Res. 2019;17(3):330.<br />
18. Plass D, Chau PYK, Thach TQ, Jahn HJ, Lai PC, Wong CM, et al.<br />
Quantifying the burden of disease due to premature mortality in<br />
Hong Kong using standard expected years of life lost. BMC Public<br />
Health. 2013;13(1):863.<br />
19. Parsonage M, Neuburger H. Discounting and health benefits.<br />
Health Econ. 1992;1(1):71–6.<br />
20. Brouwer WB, Niessen LW, Postma MJ, Rutten FF. Need for<br />
differential discounting of costs and health effects in cost<br />
effectiveness analyses. BMJ. 2005;331(7514):446–8.<br />
21. McA AK. Understanding DALYs (disability-adjusted life years). J<br />
Health Econ. 1997;16: 703730.<br />
22. Cheng L, Zhang L, Yue L, Ling J, Fan M, Yang D, et al. Expert<br />
consensus on dental caries management. Int J Oral Sci.<br />
2022;14(1):1–8.<br />
23. Shanafelt TD, Kaups KL, Nelson H, Satele DV, Sloan JA,<br />
Oreskovich MR, et al. An interactive individualized intervention to<br />
promote behavioral change to increase personal well-being in US<br />
surgeons. Ann Surg. 2014;259(1):82–8.<br />
24. Ruíz-López del Prado G, Blaya-Nováková V, Saz-Parkinson Z,<br />
Álvarez-Montero ÓL, Ayala A, Muñoz-Moreno MF, et al. Design<br />
and validation of an oral health questionnaire for preoperative<br />
anaesthetic evaluation. Braz J Anesthesiol. 2017;67(1):6–14.<br />
25. Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of<br />
inflammatory bowel disease in the UK: a single centre retrospective<br />
study. Gut. 2004;53(10):1471–8.<br />
26. Hay JW, Hay AR. Inflammatory bowel disease: costs-of-illness. J<br />
Clin Gastroenterol. 1992;14(4):309–17.<br />
27. Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE,<br />
Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the<br />
United States: 2012 update. <strong>Gastroenterology</strong>. 2012;143(5):1179-<br />
1187.e3.<br />
28. Blomqvist P, Ekbom A. Inflammatory bowel diseases: health<br />
care and costs in Sweden in 1994. Scand J Gastroenterol.<br />
1997;32(11):1134–9.<br />
29. Hay AR, Hay JW. Inflammatory bowel disease: medical cost<br />
algorithms. J Clin Gastroenterol. 1992;14(4):318–27.<br />
30. Pillai N, Dusheiko M, Maillard MH, Rogler G, Brüngger B, Bähler<br />
C, et al. The evolution of health care utilisation and costs for<br />
inflammatory bowel disease over ten years. J Crohns Colitis.<br />
2019;13(6):744–54.<br />
31. Park K, Ehrlich OG, Allen JI, Meadows P, Szigethy EM, Henrichsen<br />
K, et al. The cost of inflammatory bowel disease: an initiative<br />
from the Crohn’s & Colitis Foundation. Inflamm Bowel Dis.<br />
2020;26(1):1–10.<br />
32. van der Valk ME, Mangen M-JJ, Severs M, van der Have M,<br />
Dijkstra G, van Bodegraven AA, et al. Evolution of costs of<br />
inflammatory bowel disease over two years of follow-up. PLoS<br />
ONE. 2016;11(4): e0142481.<br />
33. van der Valk ME, Mangen M-JJ, Leenders M, Dijkstra G, van<br />
Bodegraven AA, Fidder HH, et al. Healthcare costs of inflammatory<br />
bowel disease have shifted from hospitalisation and surgery<br />
towards anti-TNFα therapy: results from the COIN study. Gut.<br />
2014;63(1):72–9.<br />
34. Stark R, König H-H, Leidl R. Costs of inflammatory bowel disease<br />
in Germany. Pharmacoeconomics. 2006;24(8):797–814.<br />
35. Juan J, Estiarte R, Colome E, Artés M, Jiménez F, Alonso J.<br />
Burden of illness of Crohn’s disease in Spain. Dig Liver Dis.<br />
2003;35(12):853–61.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
26 27
FEATURE<br />
36. Odes S, Vardi H, Riis L, Moum B, Politi P, Tsianos N, et al. Costanalysis<br />
and cost-determinants in inflammatory bowel disease.<br />
16. Sivero, L. et al. Endoscopic diagnosis and treatment of<br />
neuroendocrine<br />
<strong>Gastroenterology</strong>.<br />
tumors<br />
2005;128(4):<br />
of the digestive<br />
A324-A.<br />
system. Open Med. 11(1),<br />
37.<br />
369–373.<br />
Feagan BG,<br />
https://doi.org/10.1515/med-2016-0067<br />
Vreeland MG, Larson LR, Bala MV. Annual<br />
(2016).<br />
cost of<br />
17. Witteman, care for Crohn’s B. J., Janssens, disease: A A. payor R., Griffi perspective. oen, G. & Am Lamers, J Gastroenterol. C. B.<br />
Villous 2000;95(8):1955–60.<br />
tumours of the duodenum. An analysis of the literature with<br />
38. emphasis Le Berre on C, Ananthakrishnan malignant transformation. AN, Danese Neth. S, Singh J. Med. S, 42, Peyrin- 5 (1993).<br />
Biroulet L. Ulcerative colitis and Crohn’s disease have similar<br />
18. Levine, J. A., Burgart, L. J., Batts, K. P. & Wang, K. K. Brunner’s<br />
burden and goals for treatment. Clin Gastroenterol Hepatol.<br />
gland hamartomas: Clinical presentation and pathological features<br />
2020;18(1):14–23.<br />
of 27 cases. Am. J. Gastroenterol. 90, 290–294 (1995).<br />
39. Cohen R, Yu A, Wu E, Xie J, Mulani P, Chao J. Systematic review:<br />
19. Noguchi, the costs H. of et ulcerative al. Prevalence colitis in of Western Helicobacter countries. pylori Aliment infection rate<br />
in Pharmacol heterotopic Ther. gastric 2010;31(7):693–707.<br />
mucosa in histological analysis of duodenal<br />
40. specimens Peng YuA, from Cabanilla patients LA, with Qiong duodenal WuE, Mulani ulcer. PM, Histol. Chao Histopathol. J.<br />
35(2), The costs 169–176. of Crohn’s https://doi.org/10.14670/HH-18-142 disease in the United States and (2020) other<br />
(Epub Western 2019 countries: Jul 2). a systematic review. Curr Med Res Opin.<br />
2008;24(2):319–28.<br />
20. Singhal, S. et al. Anorectal gastrointestinal stromal tumor: A case<br />
41. Sandborn WJ, Rutgeerts P, Feagan BG, Reinisch W, Olson A,<br />
report and literature review. Case Rep. Gastrointest. Med. 2013,<br />
Johanns J, et al. Colectomy rate comparison after treatment of<br />
934875 (2013).<br />
ulcerative colitis with placebo or infliximab. <strong>Gastroenterology</strong>.<br />
21. Modlin, 2009;137(4):1250–60.<br />
I. M., Lye, K. D. & Kidd, M. A 5-decade analysis of 13,715<br />
42. carcinoid Clemente tumors. V, Aratari Cancer A, Papi 97, C, 934–959 Vernia P. (2003). Short term colectomy rate<br />
22. Bulur, and mortality A. et al. Polypoid for severe lesions ulcerative detected colitis in the last upper 40 years. Has<br />
gastrointestinal<br />
something changed?<br />
endoscopy:<br />
Dig Liver<br />
A retrospective<br />
Dis. 2016;48(4):371–5.<br />
analysis in 19560<br />
43. Barnes EL, Jiang Y, Kappelman MD, Long MD, Sandler RS, Kinlaw<br />
patients, a single-center study of a 5-year experience in Turkey.<br />
AC, et al. Decreasing colectomy rate for ulcerative colitis in the<br />
N. Clin. Istanb. 8(2), 178–185. https://doi.org/10.14744/<br />
United States between 2007 and 2016: a time trend analysis.<br />
nci.2020.16779 (2020).<br />
Inflamm Bowel Dis. 2020;26(8):1225–31.<br />
44. 23. Kostiainen, Lucendo AJ, S., De Teppo, Rezende L. & Virkkula, LC. Importance L. Papilloma of nutrition of the in<br />
oesophagus. inflammatory Report bowel of disease. a case. World Scand. J Gastroenterol: J. Thorac. Cardiovasc. WJG.<br />
Surg. 2009;15(17):2081.<br />
7(1), 95–97. https://doi.org/10.3109/14017437309139176<br />
45. (1973). Balestrieri P, Ribolsi M, Guarino MPL, Emerenziani S, Altomare<br />
24. Mandard,<br />
A, Cicala<br />
A.<br />
M.<br />
M.<br />
Nutritional<br />
et al. Cancer<br />
aspects<br />
of the<br />
in inflammatory<br />
esophagus and<br />
bowel<br />
associated<br />
diseases.<br />
Nutrients. 2020;12(2):372.<br />
lesions: Detailed pathologic study of 100 esophagectomy<br />
46. Huh J, Ackerman MS, editors. Collaborative help in chronic<br />
specimens. Hum. Pathol. 15, 660 (1984).<br />
disease management: supporting individualized problems.<br />
25. Levine, In: Proceedings J. A., Burgart, of the L. ACM J., Batts, 2012 K. Conference P. & Wang, on K. Computer K. Brunner’s<br />
gland Supported hamartomas: Cooperative Clinical Work; presentation 2012. and pathological features<br />
of 27 cases. Am. J. Gastroenterol. 90(2), 290–294 (1995).<br />
26. Ma, M. X. & Bourke, M. J. Management of duodenal polyps. Best<br />
Pract. Res. Clin. Gastroenterol. 31(4), 389–399 (2017).<br />
FEATURE<br />
47. Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, et<br />
Author contributions<br />
al. The global, regional, and national burden of inflammatory bowel<br />
Ç.E., disease M.Y.: conception, in 195 countries design, and supervision, territories, materials, 1990–2017: data a collection systematic<br />
and processing, analysis for analysis the Global and Burden interpretation, of Disease literature Study review, 2017. Lancet writer<br />
and critical Gastroenterol review. D.A., Hepatol. B.Y., 2020;5(1):17–30.<br />
K.K. O.C., İ.T., H.T.K.: materials, data<br />
48. Lo C-H, Khalili H, Song M, Lochhead P, Burke KE, Richter JM, et<br />
collection and processing, analysis and interpretation, literature review<br />
al. Healthy lifestyle is associated with reduced mortality in patients<br />
and manuscript with inflammatory supervision. bowel diseases. Clin Gastroenterol Hepatol.<br />
2021;19(1):87-95.e4.<br />
Competing interests<br />
Publisher’s Note<br />
The authors declare no competing interests.<br />
<strong>Spring</strong>er<br />
Additional<br />
Nature<br />
information<br />
remains neutral with regard to jurisdictional claims in<br />
published<br />
Correspondence<br />
maps and<br />
and<br />
institutional<br />
requests for<br />
affiliations.<br />
materials should be addressed to Ç.E.<br />
Reprints and permissions information is available at<br />
www.nature.com/reprints.<br />
Publisher’s note <strong>Spring</strong>er Nature remains neutral with regard to<br />
jurisdictional claims in published maps and institutional affi liations.<br />
Open Access This article is licensed under a Creative Commons<br />
Attribution 4.0 International License, which permits use, sharing,<br />
adaptation, distribution and reproduction in any medium or format, as<br />
long as you give appropriate credit to the original author(s) and the source,<br />
provide a link to the Creative Commons licence, and indicate if changes<br />
were made. The images or other third party material in this article are<br />
included in the article’s Creative Commons licence, unless indicated<br />
otherwise in a credit line to the material. If material is not included in the<br />
article’s Creative Commons licence and your intended use is not permitted<br />
by statutory regulation or exceeds the permitted use, you will need to<br />
obtain permission directly from the copyright holder. To view a copy of this<br />
licence, visit http:// creat iveco mmons. org/ licen ses/ by/4. 0/.<br />
© The Author(s) 2023<br />
EXACTO ®<br />
Cold Snare<br />
Purpose built to optimize the technique of<br />
cold snare polypectomy.<br />
FEATURE<br />
“The EXACTO Cold Snare has exceptional durability<br />
to use in the UGI tract, small bowel, and colon in<br />
patients with FAP. I have used a single EXACTO<br />
Cold Snare to remove almost 200 polyps in a single<br />
patient. Cold snare polypectomy provides a safe<br />
resection technique for this patient population.”<br />
- JULIE YANG, MD, FASGE, FACG, NYSGEF<br />
Director of Endoscopic GI Cancer Services and Outreach, Associate Director of<br />
the Center for Carcinoid and Neuroendocrine Tumors, Associate Professor of<br />
Medicine, Icahn School of Medicine at Mount Sinai<br />
WHY NOT WRITE FOR US?<br />
EXACTO ® Cold Snare<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
<strong>Gastroenterology</strong> <strong>Today</strong> welcomes the submission of<br />
clinical papers and case reports or news that<br />
you feel will be of interest to your colleagues.<br />
Material submitted will be seen by those working within all<br />
UK gastroenterology departments and endoscopy units.<br />
All submissions should be forwarded to info@mediapublishingcompany.com<br />
If you have any queries please contact the publisher Terry Gardner via:<br />
info@mediapublishingcompany.com<br />
GASTROENTEROLOGY TODAY - SUMMER 2023<br />
© 2023 STERIS. All rights reserved.<br />
All company and product names are trademarks of STERIS,<br />
its affiliates or related companies, unless otherwise noted.<br />
28 11<br />
29<br />
Follow us:<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong>
COMPANY NEWS<br />
COMPANY NEWS<br />
We get the gut<br />
Direct Sampling for<br />
Calprotectin Testing with<br />
New CALEX ® Patient Packs<br />
Alpha Laboratories Ltd, a leading provider<br />
of clinical diagnostics and laboratory<br />
supplies, is proud to introduce the latest<br />
advancement in faecal calprotectin<br />
extraction workflows. The CALEX ® Faecal<br />
Calprotectin Collection Kit is an innovative<br />
solution that enables patients to prepare<br />
and return their samples, revolutionising<br />
the traditional stool testing process.<br />
Stabilised samples arrive in the laboratory<br />
ready for processing and analysis.<br />
while significantly improving the speed and<br />
efficiency of stool testing workflows.<br />
Each CALEX Faecal Calprotectin Collection<br />
Kit is designed with patient convenience in<br />
mind. Discretely packaged in a businessstyle<br />
envelope, each kit contains a CALEX<br />
extraction device, patient-specific instructions<br />
for use leaflet (IFU), and a clear grip seal<br />
bag for easy return to the laboratory or GP.<br />
The IFU is written in lay terms and includes<br />
simple instructions with diagrams and links to<br />
instructional videos, ensuring ease of use for<br />
patients with varying sample consistencies.<br />
reporting ease of use and confidence in<br />
sample preparation.<br />
For hospitals and laboratories looking to<br />
enhance efficiency and streamline workflows,<br />
the CALEX Faecal Calprotectin Collection Kit<br />
offers a ready-to-use, standardised solution.<br />
For further information, please visit<br />
www.alphalabs.co.uk/CALEX-pack or contact<br />
Alpha Laboratories on 0800 38 77 32 or<br />
email marketing@alphalabs.co.uk<br />
Stay on tract.<br />
Go further.<br />
Find out why VSL#3 is recommended by gastroenterologists<br />
and dietitians to help relieve gut symptoms.<br />
In 2014, the introduction of the BÜHLMANN<br />
CALEX Cap, a groundbreaking stool<br />
extraction device, transformed laboratory<br />
workflows. Building on this success, the<br />
CALEX Collection Kit now allows patients to<br />
take control of their own sample collection<br />
process. By eliminating the need for traditional<br />
stool pots, this solution streamlines laboratory<br />
testing processes, maximising efficiency and<br />
resource utilisation.<br />
CALEX is compliant with IATA 650 (UN3373)<br />
regulations, making it suitable for air and land<br />
transportation. This opens up the possibility<br />
of outsourcing pre-analytics to patients'<br />
homes, enhancing safety for laboratories<br />
Furthermore, the stability of samples within<br />
the CALEX system is significantly enhanced<br />
compared to traditional stool samples. With<br />
stability at ambient temperature for up to<br />
7 days, and up to 15 days when stored<br />
at 2-8°C, the CALEX system provides<br />
laboratories with more consistent results and<br />
greater flexibility in testing practices.<br />
The introduction of the CALEX Faecal<br />
Calprotectin Collection Kit has been met with<br />
high levels of acceptance and compliance<br />
among both patients and healthcare<br />
professionals. Preliminary audits have shown<br />
compliance rates of up to 95%, with patients<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
A high strength probiotic containing 450 billion<br />
bacteria across 8 diverse strains<br />
Proven to survive the harsh stomach conditions,<br />
reach the gut alive and enrich the gut microbiome 1,2<br />
Over 30 clinical studies in the last 5 years alone<br />
Recommended by NHS healthcare professionals 3<br />
Help them stay on track with VSL#3.<br />
For more information, visit www.vsl3.co.uk/pages/vsl-for-hcps<br />
Publishers Comment<br />
For over 30 years thanks to trade support, we have been able to provide those working within <strong>Gastroenterology</strong> Departments<br />
and Endoscopy Units with quarterly copies of <strong>Gastroenterology</strong> <strong>Today</strong> free of charge in the knowledge that those receiving<br />
our dedicated publication enjoy having something to pick up and read during their free time. As in the current climate, return on<br />
investment appears to be the buzz word amongst suppliers, we would appreciate you mentioning <strong>Gastroenterology</strong> <strong>Today</strong><br />
when enquiring about products advertised.<br />
In respect of this current issue we would like to thank the following companies for their advertising support as without their<br />
contribution towards our print and postal costs this issue would not have been published. Alpha Laboratories, Biohit, Steris,<br />
Infai, Micro Tech, Nordic, Tillots.<br />
GASTROENTEROLOGY TODAY – SPRING <strong>2024</strong><br />
UK-VSL3-2400004. Date of Preparation: Feb <strong>2024</strong>.<br />
1. Cancello R, et al. Nutrients 2019, 11, 3011.<br />
Scan the QR code and order<br />
2. Vecchione A, et al. Front Med. 2018;5(59). doi: 10.3389/fmed.2018.00059.<br />
30 3. Nordic Pharma VSL# attribution survey, 2023.<br />
a free sample for your patient<br />
31
Helicobacter Test INFAI ®<br />
One of the most used<br />
13<br />
C-urea breath tests for the diagnosis<br />
of Hp-infections worldwide<br />
• New line of INFAI packaging and serialization according the EU’s Falsified Medicines Directive<br />
• More than 7.0 million Helicobacter Test INFAI performed worldwide<br />
• Registered in more than 40 countries worldwide<br />
• First approved Hp test for children from the ages of 3 to 11<br />
• Modified Hp test for patients taking PPIs (REFEX)<br />
• Modified Hp test for patients with atrophic gastritis<br />
• Cost-effective CliniPac Basic for hospital and GPs use<br />
INFAI UK Ltd<br />
Innovation Centre, York Science Park<br />
University Road, Heslington<br />
York YO10 5DG UK<br />
Phone: +44 1904 435 228<br />
Fax: +44 1904 435 229<br />
E-Mail: mail@infai.co.uk<br />
Web: www.infai1.com