Appendix - CNIC
Appendix - CNIC
Appendix - CNIC
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
SCIENTIFIC REPORT ´09<br />
> RESEARCH INTEREST<br />
How the genome is co-ordinately regulated during<br />
development is one of the major unanswered questions in<br />
modern biology. We are exploring this issue by means of<br />
comparative and functional approaches, with the aim of<br />
understanding how gene regulatory networks were assembled<br />
during evolution and how this determines their function.<br />
We are particularly interested in understanding the function<br />
of the gene regulatory network that controls embryonic<br />
pluripotency in the mouse embryo. Comparison with other<br />
vertebrates to determine the degree of conservation of these<br />
genes and their interactions shows that the core pluripotency<br />
factors (Oct4-Sox2-Nanog) were newly assembled into a<br />
network in the mammalian lineage and that downstream<br />
1 Cardiovascular Developmental Biology<br />
Functional genomics of embryonic<br />
pluripotency and heart development<br />
Head of Laboratory: Miguel Manzanares<br />
Postdoctoral Researchers: Susana Cañón<br />
Mª. Eva Alonso<br />
Cristina Arias<br />
13<br />
Predoctoral Researchers: Bárbara Pernaute<br />
Beatriz Fernández-Tresguerres<br />
Teresa Rayón<br />
Melisa Gómez-Velázquez<br />
target genes of this core set were recruited through the<br />
appearance of novel enhancer elements. We are also<br />
exploring the role of miRNAs as a second layer of regulation<br />
in the establishment of extraembryonic stem cell<br />
populations.<br />
Another area of interest is the potential regulatory function<br />
during development of intergenic genomic regions that have<br />
been identified with human diseases through genome-wide<br />
studies. These studies include analysis of the genomic<br />
regions associated with increased risk of type II diabetes and<br />
obesity as well as investigation into the role of p63 and its<br />
downstream regulatory network in human disease.<br />
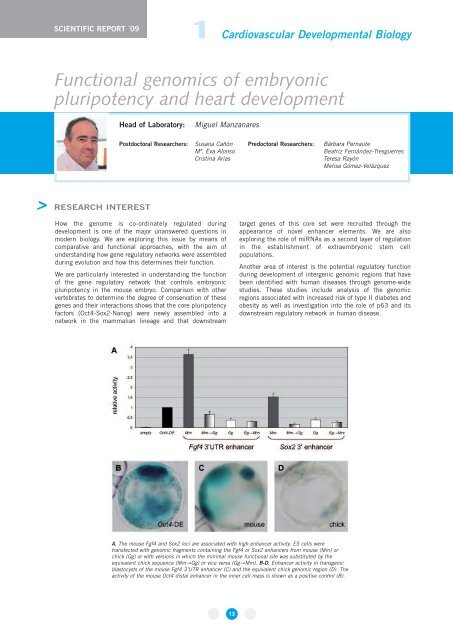
A, The mouse Fgf4 and Sox2 loci are associated with high enhancer activity. ES cells were<br />
transfected with genomic fragments containing the Fgf4 or Sox2 enhancers from mouse (Mm) or<br />
chick (Gg) or with versions in which the minimal mouse functional site was substituted by the<br />
equivalent chick sequence (Mm→Gg) or vice versa (Gg→Mm). B-D, Enhancer activity in transgenic<br />
blastocysts of the mouse Fgf4 3’UTR enhancer (C) and the equivalent chick genomic region (D). The<br />
activity of the mouse Oct4 distal enhancer in the inner cell mass is shown as a positive control (B).