insights - IMS Health
insights - IMS Health
insights - IMS Health
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
INSIGHTS | DRUG EXPOSURE MEASUREMENT<br />
Hospitalization<br />
ED/UC Visit<br />
OCS claim<br />
Any of the above<br />
Thus, in studies that do not recognize the use of nebulized SABA there is likely to be<br />
substantial misclassification of patient risk based on high SABA utilization.<br />
The present study utilized the method described by Chan, et al, 7 being inclusive of almost all<br />
MDI and nebulized SABA types identified in the dataset. Canister equivalents were<br />
determined for each claim represented. A standard SABA canister size was defined to be one<br />
containing 200 metered actuations, which encompassed more than 97% of MDI SABA claims.<br />
More than 96% of total SABA MDI claims were for albuterol; 2.2% were for pirbuterol; and<br />
1.6% were for levalbuterol. In the case of pirbuterol, whose standard dispensing contains 400<br />
actuations, claims were set to two canisters. Levalbuterol was considered to be equivalent in<br />
potency to albuterol. In cases where insufficient information was available to make the<br />
determination, canister size was imputed using days supply of medication. Thirty days supply or<br />
less was determined to be equivalent to one canister, 31 to 60 days supply as two canisters, etc.<br />
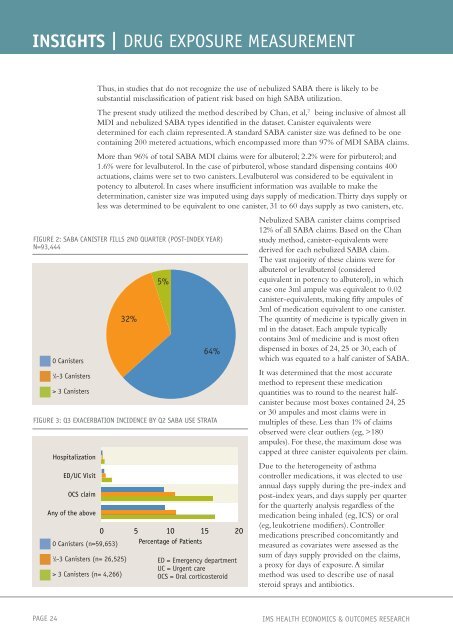
FIGURE 2: SABA CANISTER FILLS 2ND QUARTER (POST-INDEX YEAR)<br />
N=93,444<br />
0 Canisters<br />
1 ⁄2-3 Canisters<br />
> 3 Canisters<br />
0 5 10 15 20<br />
0 Canisters (n=59,653) Percentage of Patients<br />
1 ⁄2-3 Canisters (n= 26,525)<br />
> 3 Canisters (n= 4,266)<br />
32%<br />
5%<br />
64%<br />
FIGURE 3: Q3 EXACERBATION INCIDENCE BY Q2 SABA USE STRATA<br />
ED = Emergency department<br />
UC = Urgent care<br />
OCS = Oral corticosteroid<br />
Nebulized SABA canister claims comprised<br />
12% of all SABA claims. Based on the Chan<br />
study method, canister-equivalents were<br />
derived for each nebulized SABA claim.<br />
The vast majority of these claims were for<br />
albuterol or levalbuterol (considered<br />
equivalent in potency to albuterol), in which<br />
case one 3ml ampule was equivalent to 0.02<br />
canister-equivalents, making fifty ampules of<br />
3ml of medication equivalent to one canister.<br />
The quantity of medicine is typically given in<br />
ml in the dataset. Each ampule typically<br />
contains 3ml of medicine and is most often<br />
dispensed in boxes of 24, 25 or 30, each of<br />
which was equated to a half canister of SABA.<br />
It was determined that the most accurate<br />
method to represent these medication<br />
quantities was to round to the nearest halfcanister<br />
because most boxes contained 24, 25<br />
or 30 ampules and most claims were in<br />
multiples of these. Less than 1% of claims<br />
observed were clear outliers (eg, >180<br />
ampules). For these, the maximum dose was<br />
capped at three canister equivalents per claim.<br />
Due to the heterogeneity of asthma<br />
controller medications, it was elected to use<br />
annual days supply during the pre-index and<br />
post-index years, and days supply per quarter<br />
for the quarterly analysis regardless of the<br />
medication being inhaled (eg, ICS) or oral<br />
(eg, leukotriene modifiers). Controller<br />
medications prescribed concomitantly and<br />
measured as covariates were assessed as the<br />
sum of days supply provided on the claims,<br />
a proxy for days of exposure. A similar<br />
method was used to describe use of nasal<br />
steroid sprays and antibiotics.<br />
PAGE 24 <strong>IMS</strong> HEALTH ECONOMICS & OUTCOMES RESEARCH