insights - IMS Health
insights - IMS Health
insights - IMS Health
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
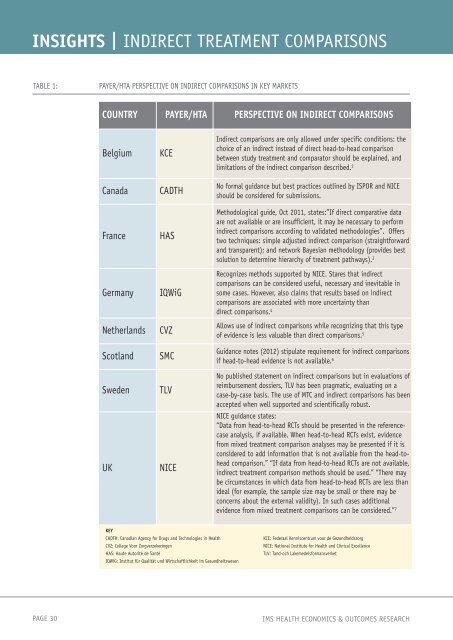
INSIGHTS | INDIRECT TREATMENT COMPARISONS<br />
TABLE 1: PAYER/HTA PERSPECTIVE ON INDIRECT COMPARISONS IN KEY MARKETS<br />
COUNTRY PAYER/HTA PERSPECTIVE ON INDIRECT COMPARISONS<br />
Belgium KCE<br />
Canada CADTH<br />
France HAS<br />
Germany IQWiG<br />
Netherlands CVZ<br />
Scotland SMC<br />
Sweden TLV<br />
UK NICE<br />
KEY<br />
CADTH: Canadian Agency for Drugs and Technologies in <strong>Health</strong><br />
CVZ: College Voor Zorgverzekeringen<br />
HAS: Haute Autorité de Santé<br />
IQWiG: Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen<br />
Indirect comparisons are only allowed under specific conditions: the<br />
choice of an indirect instead of direct head-to-head comparison<br />
between study treatment and comparator should be explained, and<br />
limitations of the indirect comparison described. 2<br />
No formal guidance but best practices outlined by ISPOR and NICE<br />
should be considered for submissions.<br />
Methodological guide, Oct 2011, states:”If direct comparative data<br />
are not available or are insufficient, it may be necessary to perform<br />
indirect comparisons according to validated methodologies”. Offers<br />
two techniques: simple adjusted indirect comparison (straightforward<br />
and transparent); and network Bayesian methodology (provides best<br />
solution to determine hierarchy of treatment pathways). 3<br />
Recognizes methods supported by NICE. Stares that indirect<br />
comparisons can be considered useful, necessary and inevitable in<br />
some cases. However, also claims that results based on indirect<br />
comparisons are associated with more uncertainty than<br />
direct comparisons. 4<br />
Allows use of indirect comparisons while recognizing that this type<br />
of evidence is less valuable than direct comparisons. 5<br />
Guidance notes (2012) stipulate requirement for indirect comparisons<br />
if head-to-head evidence is not available. 6<br />
No published statement on indirect comparisons but in evaluations of<br />
reimbursement dossiers, TLV has been pragmatic, evaluating on a<br />
case-by-case basis. The use of MTC and indirect comparisons has been<br />
accepted when well supported and scientifically robust.<br />
NICE guidance states:<br />
“Data from head-to-head RCTs should be presented in the referencecase<br />
analysis, if available. When head-to-head RCTs exist, evidence<br />
from mixed treatment comparison analyses may be presented if it is<br />
considered to add information that is not available from the head-tohead<br />
comparison.” “If data from head-to-head RCTs are not available,<br />
indirect treatment comparison methods should be used.” “There may<br />
be circumstances in which data from head-to-head RCTs are less than<br />
ideal (for example, the sample size may be small or there may be<br />
concerns about the external validity). In such cases additional<br />
evidence from mixed treatment comparisons can be considered.” 7<br />
KCE: Federaal Kenniscentrum voor de Gezondheidszorg<br />
NICE: National Institute for <strong>Health</strong> and Clinical Excellence<br />
TLV: Tand-och Lakemedelsformansverket<br />
PAGE 30 <strong>IMS</strong> HEALTH ECONOMICS & OUTCOMES RESEARCH