insights - IMS Health
insights - IMS Health
insights - IMS Health
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
INSIGHTS | IMPLEMENTING HTA IN ASIA PACIFIC<br />
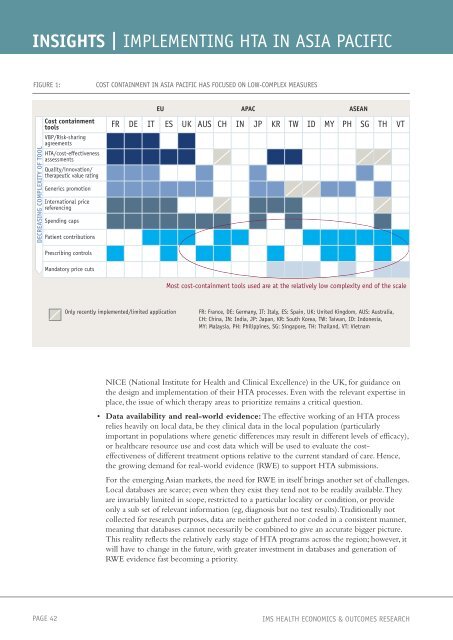
FIGURE 1: COST CONTAINMENT IN ASIA PACIFIC HAS FOCUSED ON LOW-COMPLEX MEASURES<br />
DECREASING COMPLEXITY OF TOOL<br />
Cost containment<br />
tools FR DE IT ES UK AUS CH IN JP KR TW ID MY PH SG TH VT<br />
VBP/Risk-sharing<br />
agreements<br />
HTA/cost-effectiveness<br />
assessments<br />
Quality/innovation/<br />
therapeutic value rating<br />
Generics promotion<br />
International price<br />
referencing<br />
Spending caps<br />
Patient contributions<br />
Prescribing controls<br />
Mandatory price cuts<br />
EU APAC ASEAN<br />
Most cost-containment tools used are at the relatively low complexity end of the scale<br />
Only recently implemented/limited application FR: France, DE: Germany, IT: Italy, ES: Spain, UK: United Kingdom, AUS: Australia,<br />
CH: China, IN: India, JP: Japan, KR: South Korea, TW: Taiwan, ID: Indonesia,<br />
MY: Malaysia, PH: Philippines, SG: Singapore, TH: Thailand, VT: Vietnam<br />
NICE (National Institute for <strong>Health</strong> and Clinical Excellence) in the UK, for guidance on<br />
the design and implementation of their HTA processes. Even with the relevant expertise in<br />
place, the issue of which therapy areas to prioritize remains a critical question.<br />
• Data availability and real-world evidence: The effective working of an HTA process<br />
relies heavily on local data, be they clinical data in the local population (particularly<br />
important in populations where genetic differences may result in different levels of efficacy),<br />
or healthcare resource use and cost data which will be used to evaluate the costeffectiveness<br />
of different treatment options relative to the current standard of care. Hence,<br />
the growing demand for real-world evidence (RWE) to support HTA submissions.<br />
For the emerging Asian markets, the need for RWE in itself brings another set of challenges.<br />
Local databases are scarce; even when they exist they tend not to be readily available. They<br />
are invariably limited in scope, restricted to a particular locality or condition, or provide<br />
only a sub set of relevant information (eg, diagnosis but no test results). Traditionally not<br />
collected for research purposes, data are neither gathered nor coded in a consistent manner,<br />
meaning that databases cannot necessarily be combined to give an accurate bigger picture.<br />
This reality reflects the relatively early stage of HTA programs across the region; however, it<br />
will have to change in the future, with greater investment in databases and generation of<br />
RWE evidence fast becoming a priority.<br />
PAGE 42 <strong>IMS</strong> HEALTH ECONOMICS & OUTCOMES RESEARCH