insights - IMS Health
insights - IMS Health
insights - IMS Health
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
INSIGHTS | PHARMACOGENOMICS<br />
Taking the aforementioned considerations into account, much of the relevant data is actually<br />
already routinely collected in practice. However, the sources are fragmented. Consequently,<br />
often de novo data collection projects are being developed which provide the full and<br />
complete information that is needed. However, these are expensive and time consuming.<br />
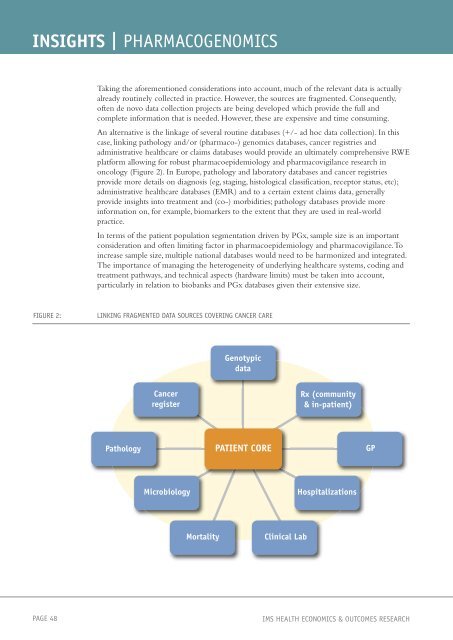
An alternative is the linkage of several routine databases (+/- ad hoc data collection). In this<br />
case, linking pathology and/or (pharmaco-) genomics databases, cancer registries and<br />
administrative healthcare or claims databases would provide an ultimately comprehensive RWE<br />
platform allowing for robust pharmacoepidemiology and pharmacovigilance research in<br />
oncology (Figure 2). In Europe, pathology and laboratory databases and cancer registries<br />
provide more details on diagnosis (eg, staging, histological classification, receptor status, etc);<br />
administrative healthcare databases (EMR) and to a certain extent claims data, generally<br />
provide <strong>insights</strong> into treatment and (co-) morbidities; pathology databases provide more<br />
information on, for example, biomarkers to the extent that they are used in real-world<br />
practice.<br />
In terms of the patient population segmentation driven by PGx, sample size is an important<br />
consideration and often limiting factor in pharmacoepidemiology and pharmacovigilance. To<br />
increase sample size, multiple national databases would need to be harmonized and integrated.<br />
The importance of managing the heterogeneity of underlying healthcare systems, coding and<br />
treatment pathways, and technical aspects (hardware limits) must be taken into account,<br />
particularly in relation to biobanks and PGx databases given their extensive size.<br />
FIGURE 2: LINKING FRAGMENTED DATA SOURCES COVERING CANCER CARE<br />
Pathology<br />
Cancer<br />
register<br />
Genotypic<br />
data<br />
PATIENT CORE<br />
Rx (community<br />
& in-patient)<br />
Microbiology Hospitalizations<br />
Mortality Clinical Lab<br />
PAGE 48 <strong>IMS</strong> HEALTH ECONOMICS & OUTCOMES RESEARCH<br />
GP