WHO Good Governance for Medicines programme: an innovative ...
WHO Good Governance for Medicines programme: an innovative ...
WHO Good Governance for Medicines programme: an innovative ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
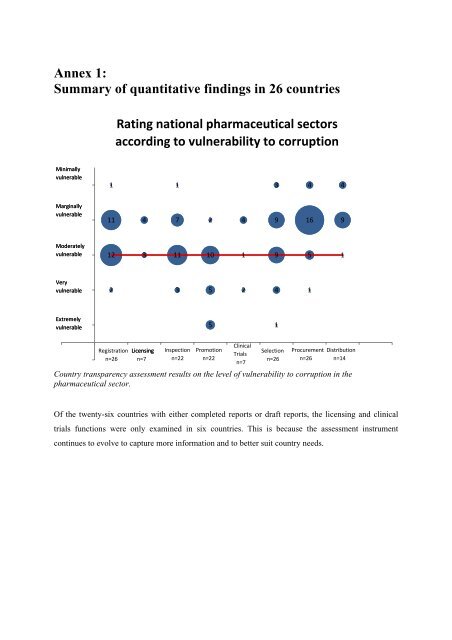
Annex 1:<br />
Summary of qu<strong>an</strong>titative findings in 26 countries<br />
Minimally<br />
vulnerable<br />
Marginally<br />
vulnerable<br />
Moderately<br />
vulnerable<br />
Very<br />
vulnerable<br />
Extremely<br />
vulnerable<br />
1<br />
11<br />
12<br />
2<br />
Registration<br />
n=26<br />
Rating national pharmaceutical sectors<br />
according to vulnerability to corruption<br />
4<br />
3<br />
Licensing<br />
n=7<br />
1<br />
7<br />
11<br />
3<br />
Inspection<br />
n=22<br />
2<br />
10<br />
5<br />
5<br />
Promotion<br />
n=22<br />
4<br />
1<br />
2<br />
Clinical<br />
Trials<br />
n=7<br />
3<br />
9<br />
9<br />
4<br />
1<br />
Selection<br />
n=26<br />
4<br />
16<br />
5<br />
1<br />
Procurement<br />
n=26<br />
4<br />
9<br />
1<br />
Distribution<br />
n=14<br />
Country tr<strong>an</strong>sparency assessment results on the level of vulnerability to corruption in the<br />
pharmaceutical sector.<br />
Of the twenty-six countries with either completed reports or draft reports, the licensing <strong>an</strong>d clinical<br />
trials functions were only examined in six countries. This is because the assessment instrument<br />
continues to evolve to capture more in<strong>for</strong>mation <strong>an</strong>d to better suit country needs.<br />
26