Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
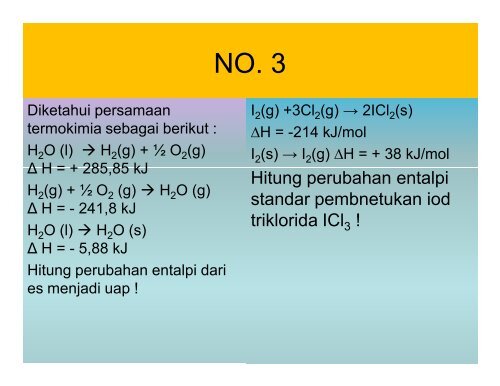
NO. 3<br />
Diketahui persamaan<br />
termokimia sebagai berikut :<br />
H 2 O (l) à H 2 (g) + ½ O 2 (g)<br />
Δ H = + 285,85 kJ<br />
H 2 (g) + ½ O 2 (g) à H 2 O (g)<br />
Δ H = - 241,8 kJ<br />
H 2 O (l) à H 2 O (s)<br />
Δ H = - 5,88 kJ<br />
Hitung perubahan entalpi dari<br />
es menjadi uap !<br />
I 2 (g) +3Cl 2 (g) → 2ICl 2 (s)<br />
∆H = -214 kJ/mol<br />
I 2 (s) → I 2 (g) ∆H = + 38 kJ/mol<br />
Hitung perubahan entalpi<br />
standar pembnetukan iod<br />
triklorida ICl 3 !