Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
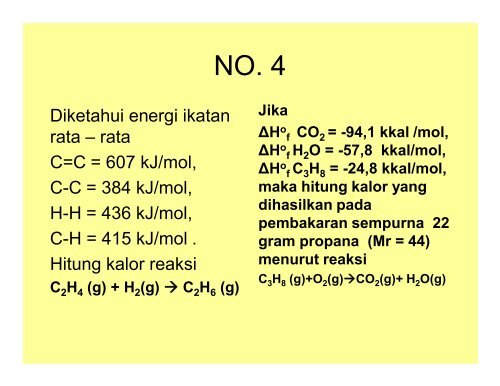
NO. 4<br />
Diketahui energi ikatan<br />
rata – rata<br />
C=C = 607 kJ/mol,<br />
C-C = 384 kJ/mol,<br />
H-H = 436 kJ/mol,<br />
C-H = 415 kJ/mol .<br />
Hitung kalor reaksi<br />
C 2 H 4 (g) + H 2 (g) à C 2 H 6 (g)<br />
Jika<br />
ΔH o f CO 2 = -94,1 kkal /mol,<br />
ΔH o f H 2 O = -57,8 kkal/mol,<br />
ΔH o f C 3 H 8 = -24,8 kkal/mol,<br />
maka hitung kalor yang<br />
dihasilkan pada<br />
pembakaran sempurna 22<br />
gram propana (Mr = 44)<br />
menurut reaksi<br />
C 3 H 8 (g)+O 2 (g)àCO 2 (g)+ H 2 O(g)