di - Skuola.net
di - Skuola.net
di - Skuola.net
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
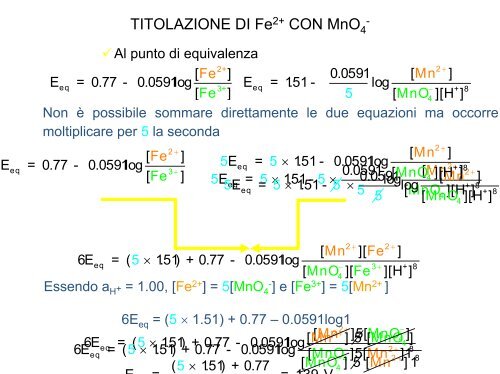
TITOLAZIONE DI Fe 2+ CON MnO 4 -<br />
Al punto <strong>di</strong> equivalenza<br />
2+<br />
2 +<br />
[ Fe ]<br />
0.0591 [ Mn ]<br />
E eq = 0.77 - 0.0591l<br />
og E 3+ eq = 1.51 - log − + 8<br />
[ Fe<br />
]<br />
5 [ M nO 4 ][H ]<br />
Non è possibile sommare <strong>di</strong>rettamente le due equazioni ma occorre<br />
moltiplicare per 5 la seconda<br />
2<br />
[ F e ]<br />
E eq = 0.77 - 0.0591log 3<br />
[ Fe ]<br />
+<br />
+<br />
2 +<br />
[ Mn ]<br />
5E = 5 × 1.51 - 0.0591log 0.0591 [ Mn ]<br />
5E 5<br />
= 5<br />
5<br />
1.51 5<br />
MnO<br />
eq − 2 + 8<br />
eq × - × 0.0591 log<br />
[ 2 +<br />
4 ][H [Mn ] ]<br />
E − + 8<br />
eq = × 1.51 - 5 × 5 lo<br />
5<br />
[ MnO g<br />
4 ][H − ] + 8<br />
[ MnO4<br />
][H ]<br />
6E = ( 5 × 1.51) + 0.77 - 0.0591log<br />
[<br />
Mn<br />
]<br />
Fe<br />
Essendo aH + = 1.00, [Fe2+ ] = 5[MnO -<br />
4 ] e [Fe3+ ] = 5[Mn2+ ]<br />
2+ 2+<br />
[ ][ ]<br />
eq − 3 + +<br />
MnO4 [ Fe<br />
][H<br />
Mn<br />
6E = ( 1.51) + 0.77 - 0.0591log<br />
6E = ( 1.51) + 0.77 - 0.0591log Mn<br />
6Eeq = (5 × 1.51) + 0.77 – 0.0591log1<br />
( 5 ×<br />
1.51) + 0.77<br />
2 +<br />
−<br />
[ 2 + ]5[ MnO −<br />
4 ]<br />
eq<br />
5 ×<br />
[ ] 5 [MnO 4 ]<br />
− 2 + 8<br />
eq 5 ×<br />
[ MnO−<br />
4 ]5[ Mn 2 + ] 1 8<br />
[MnO 4 ] 5 [Mn ] 1<br />
]<br />
8