HANDBUCH DER GUSSWERKSTOFFE - Honsel
HANDBUCH DER GUSSWERKSTOFFE - Honsel
HANDBUCH DER GUSSWERKSTOFFE - Honsel
Erfolgreiche ePaper selbst erstellen
Machen Sie aus Ihren PDF Publikationen ein blätterbares Flipbook mit unserer einzigartigen Google optimierten e-Paper Software.
28<br />
treatment conditions therefore either promote or inhibit<br />
corrosion. Local formation of a galvanic element is<br />
frequently the cause of local corrosion. This results in<br />
anodic dissolution of the base constituents.<br />
During the assembly of components comprising different<br />
materials care should be taken to ensure that no elec -<br />
tri cally conductive medium comes between them. This<br />
would otherwise produce a galvanic element and result<br />
in the electrochemical dissolution of base metal. The<br />
potential difference between the materials is the basic<br />
cause for the dissolution of the metal, the density of the<br />
anodic current being the decisive factor. Consequently,<br />
the area ratio of both material partners (electrodes)<br />
play a decisive role.<br />
According to the requirements various methods are avail -<br />
able for corrosion protection. The natural oxide skin can<br />
be replaced by considerably thicker hard anodised layers<br />
of up to 100 µm thickness. In so doing, the Si alloys be -<br />
come grey in colour and not suitable for decorative purposes.<br />
Technical anodising results in considerably better<br />
corrosion protection and in the non-compacted condition<br />
provides anex cellent base for paint systems. Further more,<br />
chemically produced coatings such as phosphating and<br />
chromating can be used for protection against corrosion.<br />
A further field of the use of paint and coating systems is<br />
also available for corrosion protection with a decorative<br />
appearance but special advice is required here.<br />
Results from standardised test methods for corrosion be -<br />
haviour can only be compared under known con ditions.<br />
Direct transfer into practical use is not admissible.<br />
MAGNESIUM<br />
In air, magnesium spontaneously covers itself with a thin<br />
layer of magnesium oxide (hydroxide). Contrary to alu-<br />
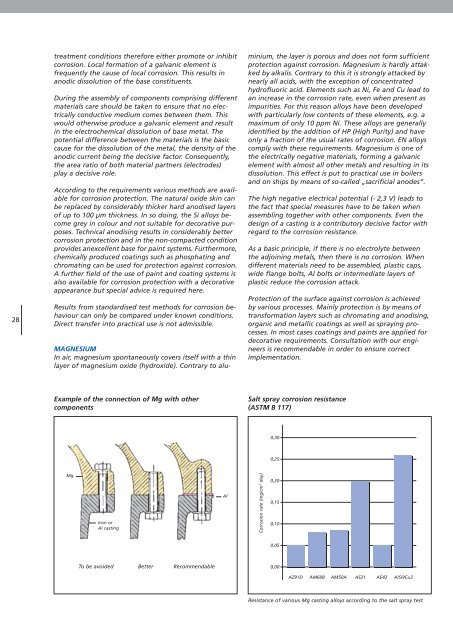
Example of the connection of Mg with other<br />
components<br />
Mg<br />
Iron or<br />
Al casting<br />
To be avoided Better Recommendable<br />
Al<br />
minium, the layer is porous and does not form suffi cient<br />
protection against corrosion. Magnesium is hardly attakked<br />
by alkalis. Contrary to this it is strongly attacked by<br />
nearly all acids, with the ex cep tion of concen trated<br />
hydrofluoric acid. Elements such as Ni, Fe and Cu lead to<br />
an increase in the corrosion rate, even when present as<br />
impurities. For this reason alloys have been developed<br />
with particularly low contents of these elements, e.g. a<br />
maximum of only 10 ppm Ni. These alloys are generally<br />
identified by the addition of HP (High Purity) and have<br />
only a fraction of the usual rates of corrosion. EN alloys<br />
comply with these requirements. Magnesium is one of<br />
the electrically negative materials, forming a galvanic<br />
element with almost all other metals and resulting in its<br />
dissolution. This effect is put to practical use in boilers<br />
and on ships by means of so-called „sacrificial anodes“.<br />
The high negative electrical potential (- 2,3 V) leads to<br />
the fact that special measures have to be taken when<br />
assembling together with other components. Even the<br />
design of a casting is a contributory decisive factor with<br />
regard to the corrosion resistance.<br />
As a basic principle, if there is no electrolyte between<br />
the adjoining metals, then there is no corrosion. When<br />
different materials need to be assembled, plastic caps,<br />
wide flange bolts, Al bolts or interme diate layers of<br />
plastic reduce the corrosion attack.<br />
Protection of the surface against corrosion is achieved<br />
by various processes. Mainly pro tection is by means of<br />
trans formation layers such as chromating and anodising,<br />
organic and metallic coatings as well as spraying pro -<br />
cesses. In most cases coatings and paints are applied for<br />
decorative require ments. Consultation with our engineers<br />
is re commendable in order to ensure correct<br />
implementation.<br />
Salt spray corrosion resistance<br />
(ASTM B 117)<br />
Corrosion rate (mg/cm 2 day)<br />
0,30<br />
0,25<br />
0,20<br />
0,15<br />
0,10<br />
0,05<br />
0,00<br />
AZ91D AM60B AM50A AS21 AE42 AlSi9Cu3<br />
Resistance of various Mg casting alloys according to the salt spray test