- Page 1 and 2: Joshua E. Goldman Candidate CIVIL E
- Page 3 and 4: DEDICATION This dissertation is ded
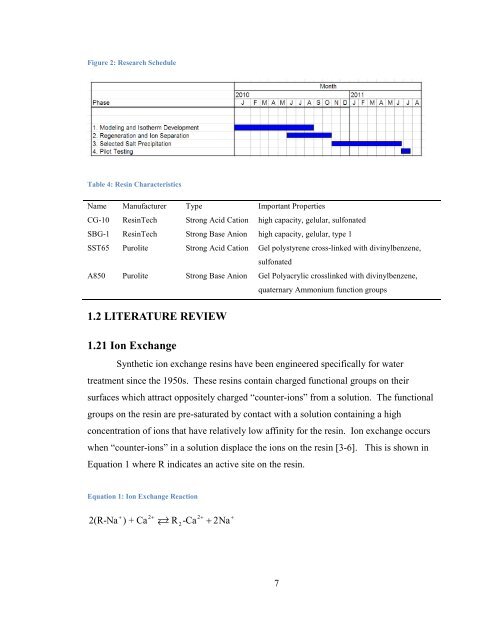
- Page 5 and 6: Selective Salt Recovery from Revers
- Page 7 and 8: TABLE OF CONTENTS ABSTRACT ........
- Page 9 and 10: 4.3 Methods........................
- Page 11 and 12: REFERENCES ........................
- Page 13 and 14: Figure 23: Predicted Magnesium Sepa
- Page 15 and 16: Figure 71: Change in Concentration
- Page 17 and 18: Table 23: Anion and Cation Regenera
- Page 19 and 20: Equation 27: Separation Factor ....
- Page 21 and 22: seawater applications in which feed
- Page 23 and 24: Each of the unit processes in the p
- Page 25: 6 Stage 2 permeate 225.00 1.72 0.00
- Page 29 and 30: complexes can form, reducing the co
- Page 31 and 32: esin phases, respectively. He found
- Page 33 and 34: segment, resulting in a smaller fra
- Page 35 and 36: Equilibrium models assume that equi
- Page 37 and 38: general enough to predict performan
- Page 39 and 40: Scaling in RO is most commonly caus
- Page 41 and 42: CHAPTER 2 MODELING One of the objec
- Page 43 and 44: Equation 18: Vector Equation to Sol
- Page 45 and 46: Figure 7: Effluent Concentration Cu
- Page 47 and 48: solution phase ion equivalents for
- Page 49 and 50: Figure 12: Comparison of Column Sna
- Page 51 and 52: CHAPTER 3 DEPENDENCE OF RESIN SELEC
- Page 53 and 54: Figure 14: Atomic Absorption Spectr
- Page 55 and 56: 2 Ca, Na 12 points Ca: 0.018 - 0.41
- Page 57 and 58: Figure 15: Calcium Separation Facto
- Page 59 and 60: used in the model are shown in Tabl
- Page 61 and 62: statistics are shown in Table 11, a
- Page 63 and 64: Figure 23: Predicted Magnesium Sepa
- Page 65 and 66: Table 12: Anion Exchange Isotherm T
- Page 67 and 68: Figure 27: Carbonate Separation Fac
- Page 69 and 70: Instead equilibrium concentrations
- Page 71 and 72: Table 13: Calculations Required to
- Page 73 and 74: was calculated. Determinations of s
- Page 75 and 76: Samples of column effluent were tak
- Page 77 and 78:
Rate mL/min % NaCl 1 25 12.5 co-cur
- Page 79 and 80:
which solutions was treated, and th
- Page 81 and 82:
Figure 34: Breakthrough Curve from
- Page 83 and 84:
SO4 Test # Predicted BVBT Measured
- Page 85 and 86:
Figure 37: Calcium Elution Curves M
- Page 87 and 88:
C/Cmax C/Cmax 1.2 0.8 0.6 0.4 0.2 1
- Page 89 and 90:
11 2.69 0.66 0.38 3.74 12 2.48 0.57
- Page 91 and 92:
CHAPTER 5 PHASE 3 -SALT PRECIPITATI
- Page 93 and 94:
were allowed to precipitate. Table
- Page 95 and 96:
5 Liquid: Initial solutions, At pH=
- Page 97 and 98:
chloride (NaCl), bassanite (Ca(SO4)
- Page 99 and 100:
can be concluded that mixing the so
- Page 101 and 102:
6 7 Figure 47: EDS Data from Experi
- Page 103 and 104:
combination of elements detected by
- Page 105 and 106:
5 6 % Precipitated % Precipitated 1
- Page 107 and 108:
5. Determine if pilot effluent recy
- Page 109 and 110:
Table 28: Legend for Schematic Diag
- Page 111 and 112:
Regen salt NaCl Regen mode Counter-
- Page 113 and 114:
Figure 51: Pilot Equipment includin
- Page 115 and 116:
Figure 54: Control Sequence at Pilo
- Page 117 and 118:
Week 2: Data collection and precipi
- Page 119 and 120:
c. At UNM, analyze for calcium, mag
- Page 121 and 122:
S6, S7 Regeneration Fluids Precipit
- Page 123 and 124:
after precipitation- no pH adjustme
- Page 125 and 126:
SBA column sample 2 cycles - week 3
- Page 127 and 128:
Sampling forms for each week, for d
- Page 129 and 130:
some startup problems. With an oper
- Page 131 and 132:
Figure 56: SEM Images of Precipitat
- Page 133 and 134:
Week 3 Week 4 Week 5 114
- Page 135 and 136:
% Composition (Atomic) % Compositio
- Page 137 and 138:
50 40 30 20 10 0 C O Na Mg Al S Cl
- Page 139 and 140:
Week 3 Week 4 Week 6 Using Jade 9.1
- Page 141 and 142:
4 2 Low 8.67 3.15 1.0366 5 2 Low 8.
- Page 143 and 144:
ambient pH conditions produced a mu
- Page 145 and 146:
without anti-scalant). PreTreat Plu
- Page 147 and 148:
Figure 68: Elution Curve for Week 5
- Page 149 and 150:
efore 1.75 bed volumes and that it
- Page 151 and 152:
Table 39: Amount of meq/g of Cl, NO
- Page 153 and 154:
hydraulics, but this resulted in wa
- Page 155 and 156:
To avoid possible precipitation of
- Page 157 and 158:
Figure 74: Normalized Permeate Flow
- Page 159 and 160:
6/24/11 9:58 AM 7.24 7.44 32.2 18.6
- Page 161 and 162:
Figure 75: Regression Relationship
- Page 163 and 164:
the inlet. This occurs because when
- Page 165 and 166:
acid cation exchange resins is appr
- Page 167 and 168:
19. Letterman, R.D. and American Wa
- Page 169 and 170:
APPENDIX 2 - UTILITY SURVEY This su
- Page 171 and 172:
152
- Page 173 and 174:
154
- Page 175 and 176:
APPENDIX 3 - MARKET ANALYSIS (BY CD
- Page 177 and 178:
Contents Section 1 Desalination Con
- Page 179 and 180:
Section 1 Desalination Concentrate
- Page 181 and 182:
UNM RO Salt Market Analysis Final R
- Page 183 and 184:
Section 2 • Concentrate Disposal
- Page 185 and 186:
Section 2 • Concentrate Disposal
- Page 187 and 188:
2.5 Concentrate Products Uses 2.5.1
- Page 189 and 190:
� Chemicals (29 percent) � Soap
- Page 191 and 192:
Section 3 Market Trends and Analysi
- Page 193 and 194:
Section 3 • Market Trends and Ana
- Page 195 and 196:
3.2 Purity Requirements and Obstacl
- Page 197 and 198:
3.2.3 Sodium Sulfate Purity Require
- Page 199 and 200:
UNM RO Salt Market Analysis Final R
- Page 201 and 202:
UNM RO Salt Market Analysis Final R
- Page 203 and 204:
3.6 Market Analysis UNM RO Salt Mar
- Page 205 and 206:
3.7 Case Studies UNM RO Salt Market
- Page 207 and 208:
Section 4 Conclusions and Recommend
- Page 209 and 210:
Section 5 References Abbazasadegan,
- Page 211 and 212:
APPENDIX 4 - FORMS USED FOR DATA CO
- Page 213 and 214:
Week 1 Cycle 1 Date Time Start Samp
- Page 215 and 216:
Week 2 Cycle 1 Date Time Start Time
- Page 217 and 218:
Week 2 Cycle 3 Date Time Start Time
- Page 219 and 220:
Week 3 Cycle 2 Date Time Start Time
- Page 221 and 222:
Week 4 Cycle 1 Date Time Start Time
- Page 223 and 224:
Week 4 Cycle 3 Date Time Start Time
- Page 225 and 226:
Week 5 Cycle 2 Date Time Start Time
- Page 227 and 228:
Week 6 Cycle 1 Date Time Start Time
- Page 229 and 230:
Week 6 Cycle 3 Date Time Start Time