Gas-Phase Reactions of Homo- and ... - Institut für Chemie
Gas-Phase Reactions of Homo- and ... - Institut für Chemie
Gas-Phase Reactions of Homo- and ... - Institut für Chemie
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1418<br />
Helvetica Chimica Acta ± Vol. 88 (2005)<br />
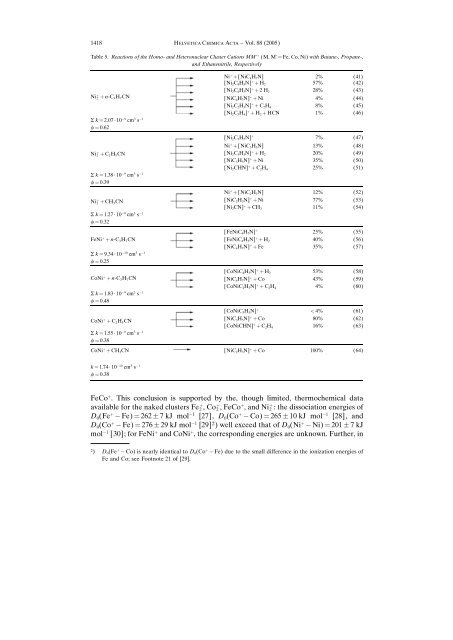
Table 5. <strong>Reactions</strong> <strong>of</strong> the <strong>Homo</strong>- <strong>and</strong> Heteronuclear Cluster Cations MM' ‡ (M, M' ˆ Fe, Co, Ni) with Butane-, Propane-,<br />
<strong>and</strong> Ethanenitrile, Respectively<br />
Ni ‡ ‡ [NiC 4H 7N] 2% (41)<br />
[Ni 2C 4H 5N] ‡ ‡ H 2 57% (42)<br />
[Ni 2C 4H 3N] ‡ ‡ 2H 2 28% (43)<br />
Ni ‡ 2 ‡ n-C 3H 7CN [NiC 4H 7N] ‡ ‡ Ni 4% (44)<br />
[Ni 2C 2H 3N] ‡ ‡ C 2H 4 8% (45)<br />
[Ni 2C 3H 4] ‡ ‡ H 2 ‡ HCN 1% (46)<br />
S k ˆ 2.07 ´ 10 9 cm 3 s 1<br />
f ˆ 0.62<br />
[Ni2C3H5N] ‡ 7% (47)<br />
Ni ‡ ‡ [NiC3H5N] 13% (48)<br />
Ni ‡ 2 ‡ C2H5CN [Ni2C3H3N] ‡ ‡ H2 20% (49)<br />
[NiC3H5N] ‡ ‡ Ni 35% (50)<br />
[Ni2CHN] ‡ S k ˆ 1.38 ´ 10<br />
‡ C2H4 25% (51)<br />
9 cm3 s 1<br />
f ˆ 0.39<br />
Ni ‡ ‡ [NiC 2H 3N] 12% (52)<br />
Ni ‡ 2 ‡ CH 3CN [NiC 2H 3N] ‡ ‡ Ni 77% (53)<br />
[Ni 2CN] ‡ ‡ CH 3 11% (54)<br />
S k ˆ 1.27 ´ 10 9 cm 3 s 1<br />
f ˆ 0.32<br />
[FeNiC 4H 7N] ‡ 25% (55)<br />
FeNi ‡ ‡ n-C 3H 7CN [FeNiC 4H 5N] ‡ ‡ H 2 40% (56)<br />
[NiC 4H 7N] ‡ ‡ Fe 35% (57)<br />
S k ˆ 9.34 ´ 10 10 cm 3 s 1<br />
f ˆ 0.25<br />
[CoNiC 4H 5N] ‡ ‡ H 2 53% (58)<br />
CoNi ‡ ‡ n-C 3H 7CN [NiC 4H 7N] ‡ ‡ Co 43% (59)<br />
[CoNiC 2H 3N] ‡ ‡ C 2H 4 4% (60)<br />
S k ˆ 1.83´ 10 9 cm 3 s 1<br />
f ˆ 0.48<br />
[CoNiC3H5N] ‡ < 4% (61)<br />
CoNi ‡ ‡ C2H5CN [NiC3H5N] ‡ ‡ Co<br />
[CoNiCHN]<br />
80% (62)<br />
‡ S k ˆ 1.55 ´ 10<br />
‡ C2H4 16% (63)<br />
9 cm3 s 1<br />
f ˆ 0.38<br />
CoNi ‡ ‡ CH3CN [NiC2H3N] ‡ ‡ Co 100% (64)<br />
k ˆ 1.74 ´ 10 10 cm 3 s 1<br />
f ˆ 0.38<br />
FeCo ‡ . This conclusion is supported by the, though limited, thermochemical data<br />
available for the naked clusters Fe ‡ 2 ,Co ‡ 2 , FeCo ‡ , <strong>and</strong> Ni ‡ 2 : the dissociation energies <strong>of</strong><br />
D 0(Fe ‡ Fe) ˆ 262 7kJ mol 1 [27], D 0(Co ‡ Co) ˆ 265 10 kJ mol 1 [28], <strong>and</strong><br />
D 0(Co ‡ Fe) ˆ 276 29 kJ mol 1 [29] 2 ) well exceed that <strong>of</strong> D 0(Ni ‡ Ni) ˆ 201 7kJ<br />
mol 1 [30]; for FeNi ‡ <strong>and</strong> CoNi ‡ , the corresponding energies are unknown. Further, in<br />
2 ) D0(Fe ‡ Co) is nearly identical to D0(Co ‡ Fe) due to the small difference in the ionization energies <strong>of</strong><br />
Fe <strong>and</strong> Co; see Footnote 21 <strong>of</strong> [29].




![Photoswitchable ionophores based on 1,3-alternate calix[4]arenes ...](https://img.yumpu.com/12290271/1/190x253/photoswitchable-ionophores-based-on-13-alternate-calix4arenes-.jpg?quality=85)