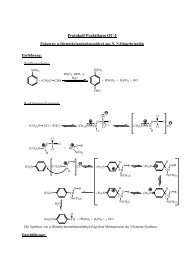
Gas-Phase Reactions of Homo- and ... - Institut für Chemie
Gas-Phase Reactions of Homo- and ... - Institut für Chemie
Gas-Phase Reactions of Homo- and ... - Institut für Chemie
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1412<br />
Model 2:<br />
D 2 ˆ f 1;1<br />
p… D2‰ dŠ†‡f1;2p…<br />
D2‰ dgŠ†‡fscr<br />
pscr… D2† KIE 2<br />
(26)<br />
H 2 ˆ f 1,2 p(H 2[dg])‡ f scr p scr(H 2) (27)<br />
HD ˆ f 1;2<br />
D 2 ˆ f 1;2<br />
p… HD‰ dgŠ†‡fscr<br />
pscr… HD†<br />
KIE<br />
p… D2‰ dgŠ†‡fscr<br />
pscr… D2† KIE 2<br />
While Models 1 <strong>and</strong> 2 reproduce the experimental data reasonably well (Table 3),<br />
some significant discrepancies remain. For equilibrium isotope effects, it is known that<br />
they are usually close to unity <strong>and</strong> much smaller than KIEs <strong>of</strong> kinetically controlled<br />
reactions, because incorporation <strong>of</strong> a heavy isotope slows down the reaction both, in the<br />
forward as well as in the reverse direction such that the overall effects on the<br />
equilibrium constant K eq are relatively small [22]. As this does not hold true for the<br />
KIEs, the latter are split into the individual contributions KIE 1,1, KIE 1,2, <strong>and</strong> KIE scr to<br />
account for the possibility that the KIE associated with the 1,1-, 1,2-elimination, <strong>and</strong> the<br />
losses <strong>of</strong> H 2/HD/D 2 after equilibration, respectively, differ. With this refined approach,<br />
Eqns. 24 ± 26 <strong>and</strong> 27 ± 29 change into Eqns. 30 ± 32 (Model 3) <strong>and</strong> 33 ± 35 (Model 4),<br />
respectively, <strong>and</strong> the results <strong>of</strong> these calculations are in much better agreement with the<br />
experimental data (Table 3). The best fits obtained <strong>and</strong> the respective parameter sets<br />
for each model are given in Table 4.<br />
(28)<br />
(29)<br />
(3<br />
Model 3:<br />
D2 ˆ f1,1 p(H2[d])‡ f1,2 p(H2[dg])‡ fscr pscr(H2) 0)<br />
HD ˆ f 1;1<br />
p… HD‰ dŠ†<br />
KIE 1;1<br />
Helvetica Chimica Acta ± Vol. 88 (2005)<br />
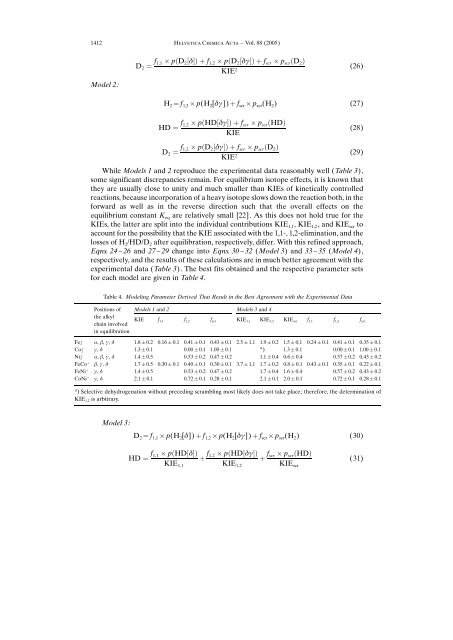
Table 4. Modeling Parameter Derived That Result in the Best Agreement with the Experimental Data<br />
Positions <strong>of</strong><br />
the alkyl<br />
chain involved<br />
in equilibration<br />
Models 1 <strong>and</strong> 2 Models 3 <strong>and</strong> 4<br />
KIE f1,1 f1,2 fscr KIE1,1 KIE1,2 KIEscr f1,1 f1,2 fscr Fe ‡ 2 a, b, g, d 1.8 0.2 0.16 0.1 0.41 0.1 0.43 0.1 2.5 1.1 1.9 0.2 1.5 0.1 0.24 0.1 0.41 0.1 0.35 0.1<br />
Co ‡ 2 g, d 1.3 0.1 0.00 0.1 1.00 0.1<br />
a ) 1.3 0.1 0.00 0.1 1.00 0.1<br />
Ni ‡ 2 a, b, g, d 1.4 0.5 0.53 0.2 0.47 0.2 1.1 0.4 0.6 0.4 0.57 0.2 0.43 0.2<br />
FeCo ‡ b, g, d 1.7 0.5 0.30 0.1 0.40 0.1 0.30 0.1 3.7 1.1 1.7 0.2 0.8 0.1 0.43 0.1 0.35 0.1 0.22 0.1<br />
FeNi ‡ g, d 1.4 0.5 0.53 0.2 0.47 0.2 1.7 0.4 1.6 0.4 0.57 0.2 0.43 0.2<br />
CoNi ‡ g, d 2.1 0.1 0.72 0.1 0.28 0.1 2.1 0.1 2.0 0.1 0.72 0.1 0.28 0.1<br />
a ) Selective dehydrogenation without preceding scrambling most likely does not take place; therefore, the determination <strong>of</strong><br />
KIE1,2 is arbitrary.<br />
‡ f 1;2<br />
p… HD‰ dgŠ†<br />
‡ fscr KIE 1;2<br />
pscr… HD†<br />
KIE scr<br />
(31)




![Photoswitchable ionophores based on 1,3-alternate calix[4]arenes ...](https://img.yumpu.com/12290271/1/190x253/photoswitchable-ionophores-based-on-13-alternate-calix4arenes-.jpg?quality=85)