CONTRIBUTION TO CINCHONA ALKALOIDS CHEMISTRY
CONTRIBUTION TO CINCHONA ALKALOIDS CHEMISTRY
CONTRIBUTION TO CINCHONA ALKALOIDS CHEMISTRY
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Ph.D. Thesis Abstract Contribution to cinchona alkaloids chemistry<br />
.<br />
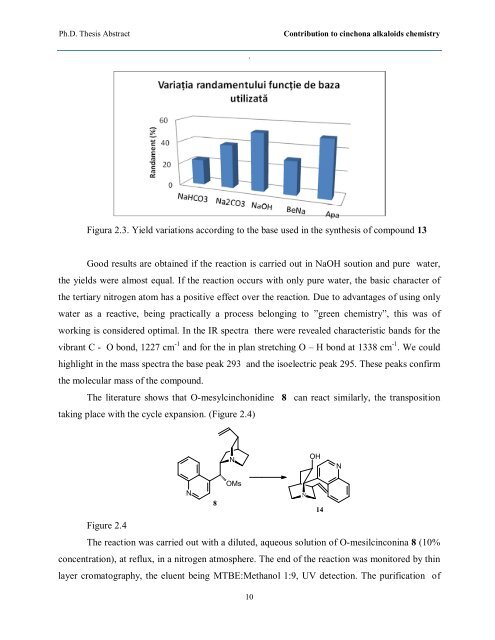
Figura 2.3. Yield variations according to the base used in the synthesis of compound 13<br />
Good results are obtained if the reaction is carried out in NaOH soution and pure water,<br />
the yields were almost equal. If the reaction occurs with only pure water, the basic character of<br />
the tertiary nitrogen atom has a positive effect over the reaction. Due to advantages of using only<br />
water as a reactive, being practically a process belonging to ”green chemistry”, this was of<br />
working is considered optimal. In the IR spectra there were revealed characteristic bands for the<br />
vibrant C - O bond, 1227 cm -1 and for the in plan stretching O – H bond at 1338 cm -1 . We could<br />
highlight in the mass spectra the base peak 293 and the isoelectric peak 295. These peaks confirm<br />
the molecular mass of the compound.<br />
The literature shows that O-mesylcinchonidine 8 can react similarly, the transposition<br />
taking place with the cycle expansion. (Figure 2.4)<br />
Figure 2.4<br />
N<br />
8<br />
N<br />
OMs<br />
The reaction was carried out with a diluted, aqueous solution of O-mesilcinconina 8 (10%<br />
concentration), at reflux, in a nitrogen atmosphere. The end of the reaction was monitored by thin<br />
layer cromatography, the eluent being MTBE:Methanol 1:9, UV detection. The purification of<br />
10<br />
N<br />
OH<br />
14<br />
N