CONTRIBUTION TO CINCHONA ALKALOIDS CHEMISTRY
CONTRIBUTION TO CINCHONA ALKALOIDS CHEMISTRY
CONTRIBUTION TO CINCHONA ALKALOIDS CHEMISTRY
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Ph.D. Thesis Abstract Contribution to cinchona alkaloids chemistry<br />
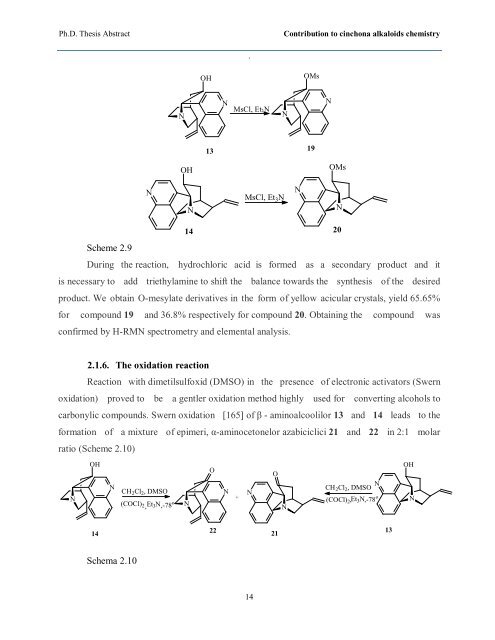
Scheme 2.9<br />
N<br />
N<br />
OH<br />
N<br />
14<br />
OH<br />
13<br />
N<br />
.<br />
MsCl, Et 3N<br />
MsCl, Et 3N<br />
During the reaction, hydrochloric acid is formed as a secondary product and it<br />
is necessary to add triethylamine to shift the balance towards the synthesis of the desired<br />
product. We obtain O-mesylate derivatives in the form of yellow acicular crystals, yield 65.65%<br />
for compound 19 and 36.8% respectively for compound 20. Obtaining the compound was<br />
confirmed by H-RMN spectrometry and elemental analysis.<br />
2.1.6. The oxidation reaction<br />
Reaction with dimetilsulfoxid (DMSO) in the presence of electronic activators (Swern<br />
oxidation) proved to be a gentler oxidation method highly used for converting alcohols to<br />
carbonylic compounds. Swern oxidation [165] of β - aminoalcoolilor 13 and 14 leads to the<br />
formation of a mixture of epimeri, α-aminocetonelor azabiciclici 21 and 22 in 2:1 molar<br />
ratio (Scheme 2.10)<br />
N<br />
OH<br />
14<br />
N<br />
Schema 2.10<br />
CH 2Cl 2, DMSO<br />
(COCl) 2, Et 3N,-78 o<br />
N<br />
O<br />
22<br />
N<br />
+<br />
N<br />
14<br />
O<br />
21<br />
N<br />
N<br />
N<br />
OMs<br />
19<br />
N<br />
OMs<br />
N<br />
20<br />
N<br />
CH2Cl2, DMSO<br />
(COCl) 2,Et 3N,-78 o<br />
13<br />
OH<br />
N