CONTRIBUTION TO CINCHONA ALKALOIDS CHEMISTRY
CONTRIBUTION TO CINCHONA ALKALOIDS CHEMISTRY
CONTRIBUTION TO CINCHONA ALKALOIDS CHEMISTRY
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Ph.D. Thesis Abstract Contribution to cinchona alkaloids chemistry<br />
Nr.<br />
Exp.<br />
1<br />
2<br />
3<br />
Faza mobilă Timp<br />
Compoziția<br />
Tampon fosfat(pH 3):<br />
acetonitril 50:50<br />
Tampon fosfat(pH 3):<br />
acetonitril 50:50<br />
Tampon fosfat(pH 3):<br />
acetonitril 50:50<br />
.<br />
Debit<br />
(ml/min)<br />
34<br />
retenție<br />
(min)<br />
Aria<br />
picului<br />
(mV·s)<br />
0,5 9,58 87743 915<br />
0,7 5,25 79543 528<br />
1,0 3,62 64211 326<br />
Capacitate<br />
(k)<br />
Mobile phase flow for an optimum separation is 0.5 ml / minute. Increasing the mobile<br />
phase flow leads to lower retention time and thus determination time, but separation is<br />
not appropriate.<br />
Experiments resulted in the establishment of the optimal mobile phase composition which<br />
allows efficient separation. It consists of: phosphate buffer: acetonitrile, 50:50 v/v, containing<br />
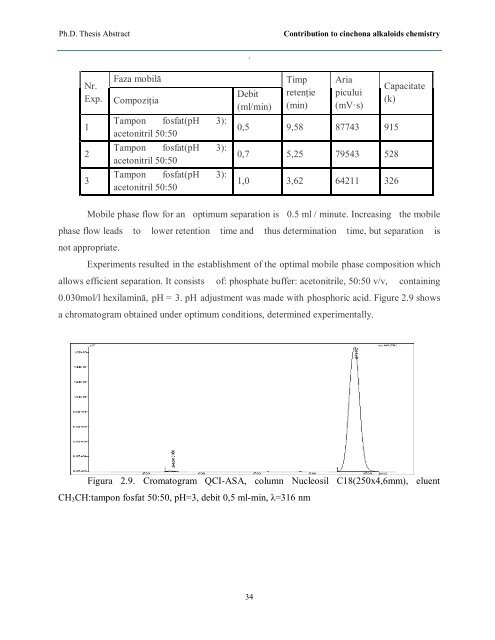
0.030mol/l hexilamină, pH = 3. pH adjustment was made with phosphoric acid. Figure 2.9 shows<br />
a chromatogram obtained under optimum conditions, determined experimentally.<br />
Figura 2.9. Cromatogram QCI-ASA, column Nucleosil C18(250x4,6mm), eluent<br />
CH3CH:tampon fosfat 50:50, pH=3, debit 0,5 ml-min, λ=316 nm