Biodentine
Biodentine
Biodentine
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
20<br />
❹ Outstanding<br />
biocompatibility<br />
From a regulatory point of view, <strong>Biodentine</strong> is a calcium silicate based material, used<br />
for crown and root dentine repair treatment, involving external contact for a period of<br />
more than 30 days. The biocompatibility tests required for the preclinical evaluation of<br />
dental products followed the guideline ISO 7405 - 2008.<br />
The following sections evaluate the compliance with this standard for the tests carried<br />
out on <strong>Biodentine</strong>. It is considered a device with external contact, for long-term tissue<br />
contact (>30 days). In certain indications (radicular, apical obstruction and repair of the<br />
pulpal floor), it can be considered an implanted system, according to the ISO<br />
classification.<br />
All biocompatibility tests were carried out on the final product <strong>Biodentine</strong>.<br />
4.1 - Cytotoxicity tests (ISO 7405, ISO 10993-5)<br />
Different cytotoxicity tests carried out on <strong>Biodentine</strong> are reported.<br />
The first study was performed on human pulpal fibroblasts (human wisdom tooth),<br />
comparing <strong>Biodentine</strong>, calcium hydroxide and MTA (Dycal ® , Dentsply and ProRoot ®<br />
MTA Dentsply). The cell viability was determined by MTT incorporation (About, 2003b).<br />
Results showed <strong>Biodentine</strong> was non cytotoxic like MTA, whereas the undiluted cement<br />
Dycal ® induced 22 % of cytotoxicity (Table. 1).<br />
Product Cell death (%)<br />
<strong>Biodentine</strong> 0±8<br />
MTA 0±9<br />
CaOH 22±10<br />
Table 1. Cell death after Dycal ® , MTA<br />
and BIODENTINE contact.<br />
Control<br />
<strong>Biodentine</strong><br />
(4 weeks)<br />
MTA<br />
(4 weeks)<br />
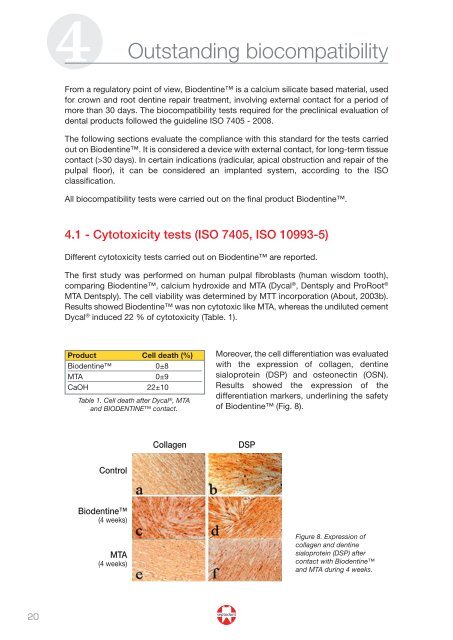
Collagen DSP<br />
Moreover, the cell differentiation was evaluated<br />
with the expression of collagen, dentine<br />
sialoprotein (DSP) and osteonectin (OSN).<br />
Results showed the expression of the<br />
differentiation markers, underlining the safety<br />
of <strong>Biodentine</strong> (Fig. 8).<br />
Figure 8. Expression of<br />
collagen and dentine<br />
sialoprotein (DSP) after<br />
contact with <strong>Biodentine</strong><br />
and MTA during 4 weeks.